New Advances Pose New Challenges to Bioproduction
A Guest Blog by Stacey Garcia, Ph.D. Product Manager, Essential Pharmaceuticals
The sales of biologic drugs have surpassed $125 billion. As the market has grown, increased competition between companies making therapeutics for the same target, such as TNF-α, is occurring. Additional price pressure has been brought on by the entry of biosimilars, furthering the need to reduce manufacturing costs. One way to reduce costs is to increase manufacturing productivity. Many tools exist to increase productivity, and in each case media optimization is required as part of the program. Often scientists focus on maximizing protein production, but there are potential consequences for focusing only on increasing protein titer. In this article, we discuss some of the available tools for increasing productivity, the complexities associated with reducing biomanufacturing expenses and potential solutions that are available to address these issues.
An opportunity for additional efficiencies in biomanufacturing
While the biologics market is expanding, the pressures to reduce cost are growing. In order to adjust to decreasing price, manufacturers have been managing costs. One way that manufacturers can reduce cost is to increase productivity – or amount of protein titer per volume – in a bioreactor. By increasing protein titer, manufacturers can increase their overall protein output. However, some manufacturers may already be meeting demands and would not see a cost decrease associated with increasing protein output. Therefore, some manufacturers instead aim to shorten the time to desired protein output, which reduces cost by shortening bioreactor run times.
A growing field of interest focuses on increasing protein titer through genetic manipulation of cell lines. For example, Thermo Fisher developed ExpiCHO, a cell line genetically engineered to enable increased protein yield in transiently transfected cells, reducing the need to transition through HEK293 during discovery, only to then switch to CHO for bioproduction. But even as new tools become available, media optimization is still an integral step for process improvement. To accomplish this, manufacturers can choose from several commercially-available mediums and supplements to optimize protein expression. Supplements can be added as a one-time boost at the start of the culture, or as a feed added recurrently during the production period. In the last few decades, average titers across the industry have greatly increased, from <0.5 g/L in the 1980s to upwards of 3 g/L today, and it is not uncommon to see titers of > 5 g/L (1).
However, as manufacturers continue to optimize their bioproduction processes to achieve increased protein titer or reach their output goals with shorter runs, they must ensure that protein efficacy is not negatively impacted. Other challenges to increasing yield include scalability, downstream efficiency, and changes to the metabolic profile. A key to optimizing protein production without impacting protein quality is managing the health of the cells in culture, specifically cell metabolism. It is important to evaluate the culture media scheme to ensure that cells have access to critical components, such as lipids and cholesterol, which are necessary for cell metabolism.
Lipids and cholesterol have many important functions in the cell. They are the building blocks of cell membranes, act as messengers to send information between cells (cell signaling), and can be an important energy source. Specifically, lipids and cholesterol are components of the outer membrane as well as the endoplasmic reticulum, Golgi apparatus, and other organelles. The endoplasmic reticulum and Golgi apparatus are involved in protein translation processing, including folding and glycosylation. Fatty acids are a type of lipid that can be synthesized by cells, but a select few fatty acids (called essential fatty acids) cannot be synthesized in high enough quantities by cells and must be obtained from the external environment. Although cells can synthesize cholesterol and some fatty acids on their own, providing lipids from an outside source can improve cell growth and metabolism in culture. This then allows the molecules needed for lipogenesis (eg. ATP) to be available for other critical metabolic processes.
In the absence of bioavailable fatty acids and cholesterol, cells in culture will use energy for membrane synthesis, which can negatively impact cell health and protein modification and secretion. For bioproduction engineers, this can be an issue as protein synthesis, trafficking, and processing may also be impacted. The end result can include protein misfolding, altered glycosylation profile, altered functionality, and other negative impacts. A media scheme that provides lipids as a feed or supplement recurrently throughout the bioproduction process can improve titer and potentially shorten bioreactor runs reducing cost for manufacturers.
A solution that provides lipids in serum-free conditions
Cell-Ess is a chemically-defined, animal component-free medium developed by Essential Pharmaceuticals, LLC and used by bioproduction engineers to increase protein yield without impacting protein quality. This novel supplement can be added to previously optimized serum-free media for greater protein yield or used as a culture media in place of FBS or other media.
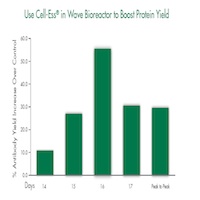
In recent studies, Cell-Ess used as an initial supplement at 1% (v/v) in a serum-free system increased monoclonal antibody in raw titer by 25% and on a per cell basis by 40%. Cell-Ess used as a feed at 5% (v/v) extended the window for peak production between 1 and 2 days. The metabolic profile of cells provided Cell-Ess was similar to that of the control groups. In addition, Cell-Ess used as a feed in a single use Wave Bag generated 25% more antibodies compared to the control.

By making lipids available to cells, Cell-Ess enables scientists to increase protein titer without impacting functionality. Further, increase in production on a per cell basis avoids a bottleneck downstream during protein purification. By increasing peak production, Cell-Ess enables scientists to either take their runs down early because they hit their goal amount of protein earlier, or enable fewer runs per year. Both of these scenarios can provide a compelling cost benefit. Cell-Ess increases protein yield in both shake flasks and single-use bioreactors, providing bioproduction engineers flexibility in material use and scalability. Finally, these results are reproducible, demonstrated in duplicate and triplicate and at various Cell-Ess concentrations, providing confidence that Cell-Ess can be utilized in many different applications.
Bringing it all together
Increased competition in the biologics industry will continue to put pressure on manufacturers to reduce costs associated with biomanufacturing. Innovation to increase protein yield is one way that manufacturers are achieving improved efficiencies. As bioproduction engineers move to single-use bioreactors, constant perfusion, or other means to streamline their processes, they will continue to evaluate media optimization as a key strategy to improve efficiency and reduce cost. However, improvements in protein productivity due to media optimization should not negatively impact efficacy and downstream efficiency. A factor in cell health, and subsequently protein functionality, is bioavailability of lipids, which is a challenge in serum-free systems. Essential Pharmaceuticals has developed Cell-Ess lipid supplement to improve protein productivity without impacting functionality, even in single-use bioreactors.
Essential Pharmaceuticals has presented detailed technical content regarding the performance of Cell-Ess at scientific congresses. These data evaluate protein yield, glycosylation patterns, metabolite profiles, scalability, and bioavailability of lipids. Click here to view recent data on the impact of Cell-Ess on protein productivity.
References
(1) Rader RA and Langer ES. Biopharmaceutical Manufacturing: Historical and Future Trends in Titers, Yields, and Efficiency in Commercial-Scale Bioprocessing. Bioprocessing Journal 2015 13:4, 47-54.