
New Small Molecule Cocktail Provides Effective hPSC Cytoprotection to Increase Efficiency in Stem Cell Workflows
Human pluripotent stem cells (hPSCs) have an extensive capacity for self-renewal and the ability to differentiate into virtually any cell type in the body. These cells represent an exciting paradigm for disease modeling, drug discovery and regenerative medicine. However, a significant challenge to the clinical translation of hPSCs is the inherent sensitivity of the cells to their in vitro environment, where poor survival during both routine culture and cell manipulations, especially involving single-cell dissociation, considerably limits the efficiency and scalability of this promising technology.
Often, stem cell labs develop their own in-house strategies to compensate for poor cell survival, leading to a range of variability in culture strategies and low reproducibility. In 2007, a key publication by Watanabe et al.1 described the addition of the small molecule Y27632, a rho-associated kinase (ROCK) inhibitor, to the culture media to improve hPSC survival. Y27632 supplementation is now very common in stem cell labs, but even with the addition of Y27632, low efficiency and significant cell death remains an issue across many hPSC applications. The lack of standardization in culture techniques is another major hurdle in the field, where efficient and scalable hPSC workflows are required to support their long-term culture and downstream differentiation to fully realize their potential in the clinic.
Recently, researchers at the National Institutes of Health (NIH) sought to overcome these challenges with an optimized and comprehensive solution to improve cell survival of hPSCs with protocols translatable to clinical use. Chen et al. published results from a targeted high-throughput screen of >15,000 small molecule compounds to identify a media supplementation strategy known as CEPT, a combination of Chroman 1, Emricasan, Polyamine solution, and Trans-ISRIB, which greatly enhances the viability and expansion of genetically stable hPSCs in culture, as well as improve the functionality of derived cell types2. Chroman 1, a selective ROCK inhibitor found to be more potent and more selective than the widely used Y‑27632 compound, plays a critical role in the CEPT supplementation strategy. In subsequent combinatorial screening, additional compounds, including the pan-caspase inhibitor Emricasan, integrated stress response (ISR) inhibitor Trans-ISRIB, and a polyamine solution, were found to work synergistically with Chroman 1 to further enhance hPSC survival after manipulation.
The efficacy of the CEPT cocktail was evaluated for long-term single-cell passaging of several different hPSC lines, where cells were exposed to the compounds for 24 hours at each passage. After extended serial single-cell passaging, the hPSC cultures showed the hallmark characteristics of healthy undifferentiated hPSCs – the cultures displayed typical morphology, high expression of pluripotency markers, maintained normal karyotypes, and retained their ability for trilineage differentiation.
The authors found the CEPT small molecule cocktail to be effective and safe for use in long-term stem cell culture, as well as beneficial in additional hPSC applications, including:
- Single-cell cloning and gene editing
- iPSC reprogramming
- Embryoid body (EB) and organoid formation
- Cryopreservation and cell banking
The published results across a variety of applications show that the CEPT cocktail effectively addresses key challenges in translational stem cell research by overcoming cellular stressors inherent in stem cell workflows that can induce DNA damage and cause cell death. The CEPT combination offers a standardized and powerful chemical platform to support efficient hPSC culture with wide-ranging implications for tissue engineering, regenerative medicine, and drug development.
Since its initial publication, excitement in the potential of the CEPT small molecule cocktail has sparked scientists to implement its use in a variety of research protocols, including enabling automated hPSC culture3, optimizing EB and organoid models (i.e., brain, gut, and kidney)4, improving cell survival in directed neural differentiation protocols (i.e., radial glial and astrocytes5; and sensory neurons6), and supporting research to better understand placental development and function7. These projects are demonstrating the efficacy and versatility of the cocktail to promote optimal cytoprotection and cell survival of hPSCs across several key applications with many more studies underway.
Captivate Bio Offers New CET Cocktail
In response to growing interest in the publication from Chen et al. and the significant potential of the small molecule cocktail to improve the efficiency of stem cell applications, Captivate Bio has released a commercially available CET Cocktail small molecule kit as a cost-effective and convenient solution for researchers to enhance overall cytoprotection, survival, and quality of hPSC cultures under stressful conditions. The CET Cocktail kit is comprised of key small molecules in the published CEPT recipe, containing Chroman 1, Emricasan, and Trans-ISRIB. While the CET Cocktail kit is intended to be used according to the publication’s protocol, each small molecule is packaged individually to allow researchers the ability for further optimization of specific protocols and applications, if desired.
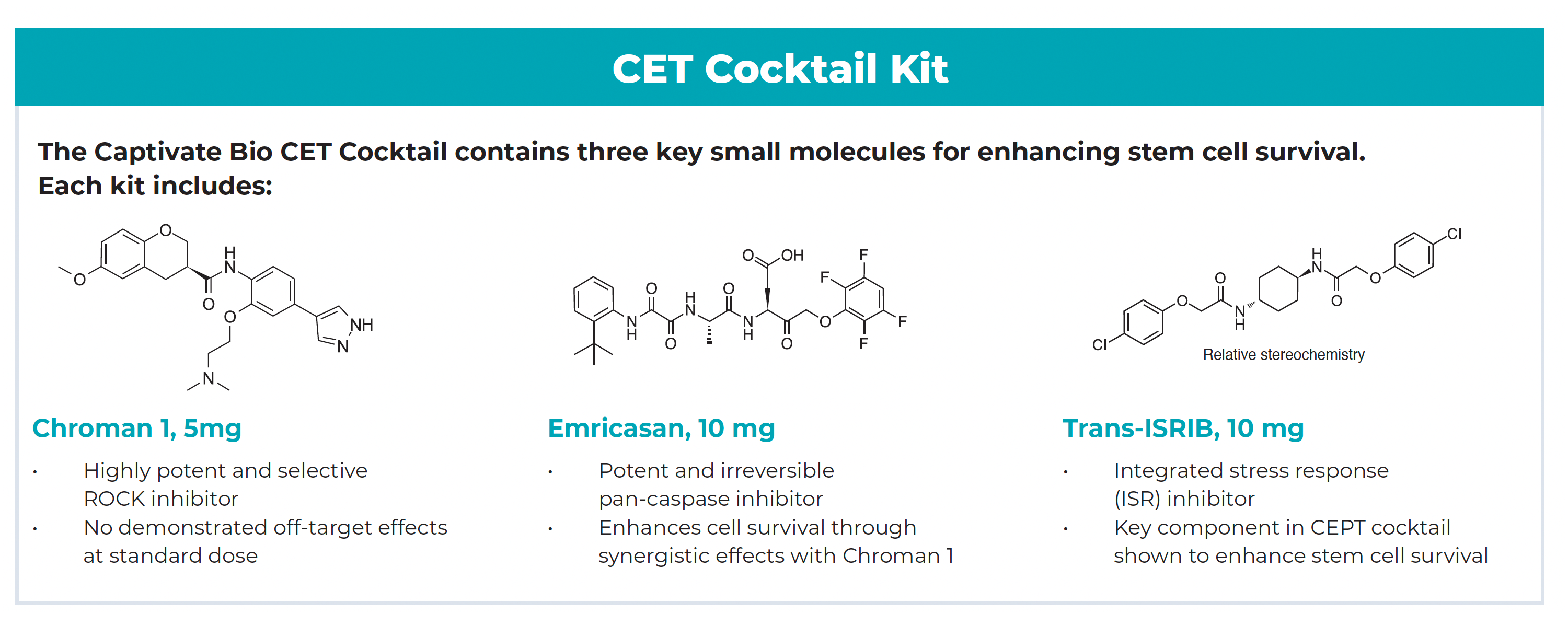
When combined with the polyamine solution (per the NIH publication protocol), the small molecules in the CET Cocktail provide reliable and comprehensive cytoprotection of hPSCs to overcome inherent low survival efficiencies observed in critical stem cell workflows. Key advantages of CET Cocktail include improved survival of dissociated cells in culture, higher yield of viable hPSC clones, and demonstration to be efficacious and safe long-term use with hPSC cultures. Moreover, the CET Cocktail is compatible with commonly used hPSC cell lines, culture media formulations, and substrates to support a diversity of stem cell techniques to accelerate innovation and drive scalable bioproduction for successful clinical translation of new therapies.
For more information on the CET Cocktail, please visit: https://captivatebio.com/cet-cocktail.html
For collaboration opportunities around the CET Cocktail or other stem cell applications, contact the Captivate Bio Technology team and apply for the Collaboration Program by visiting: https://captivatebio.com/partnerships
References
- Watanabe, K., Ueno, M., Kamiya, D. et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 25, 681–686 (2007). https://doi.org/10.1038/nbt1310
- Chen Y, Tristan CA, Chen L, et al. A versatile polypharmacology platform promotes cytoprotection and viability of human pluripotent and differentiated cells. Nat Methods. 2021;18(5):528-541. doi:10.1038/s41592-021-01126-2
- Tristan CA, Ormanoglu P, Slamecka J, et al. Robotic high-throughput biomanufacturing and functional differentiation of human pluripotent stem cells. Stem Cell Reports. 2021;16(12):3076-3092. doi:10.1016/j.stemcr.2021.11.004
- Ryu S, Weber C, Chu PH, Tristan CA, et al. Enhancing the Fitness of Embryoid Bodies and Organoids by Chemical Cytoprotection. bioRxiv doi: https://doi.org/10.1101/2022.03.21.485225
- Deng T, Tristan CA, Weber C et al. Scalable Generation of Pseudo-Unipolar Sensory Neurons from Human Pluripotent Stem Cells. bioRxiv doi: https://doi.org/10.1101/2022.03.24.485622
- Jovanovic VM, Malley C, Tristan CA et al. Directed Differentiation of Human Pluripotent Stem Cells into Radial Glia and Astrocytes Bypasses Neurogenesis. bioRxiv doi: https://doi.org/10.1101/2021.08.23.457423
- Slamecka J, Tristan CA, Seungmi Ryu S et al. A Comprehensive Roadmap of Human Placental Development in vitro. bioRxiv doi: https://doi.org/10.1101/2022.04.07.487558