
Opportunities for Leveraging Biomedical Innovations in the Development of the Clean Meat Industry
Motivated by an interest in alleviating the immense environmental impact of intensive animal agriculture, the past two years have witnessed a surge in efforts to develop bioengineered products that directly replicate the cellular composition of meat. This wouldn’t be the first time technology developed in biomedicine has made the leap to the food industry. As demonstrated by the successful cross-application of recombinant protein production technology from human insulin synthesis to widespread food enzyme and ingredient production, the potential to revolutionize food product development with biomedical research is significant but largely unexplored. Scientists are now beginning to tap into this potential by utilizing animal cell culture advancements to make products that are referred to as clean meat because they are free of the environmental, public health, and animal welfare consequences of animal rearing, slaughter, and antibiotic use.
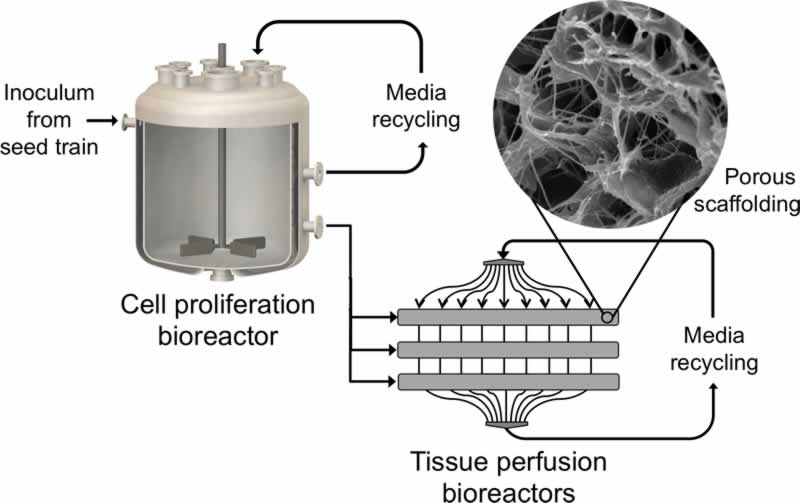
The first step in making clean meat products is the proliferation of cells derived from meat-relevant species in an animal-free cell culture medium. To create structurally complex meat analogues, the expanded cells can be subsequently seeded onto a biodegradable or edible scaffold that supports differentiation and maturation into multiple cell types. Using a combination of the scaffold parameters and other environmental conditions including the introduction of specific growth factors, these precursor cells can be directed towards differentiation into muscle cells, fat cells, and connective tissue to closely mimic the ratio and spatial organization of cell types found in the desired meat product. On a large scale, this method of meat cultivation could offer consumers the option to continue enjoying meat products at a fraction of the environmental, animal welfare, and public health impacts posed by current animal agricultural practices.
In the article, “Opportunities for applying biomedical production and manufacturing methods to the development of the clean meat industry,” Dr. Liz Specht and her colleagues at The Good Food Institute — a nonprofit organization that fosters the development of alternatives to industrialized animal agriculture — explore the critical technology sectors necessary for the commercialization of clean meat. The authors detail the current challenges confronted by the clean meat industry and discuss how to address these challenges by leveraging research from other large-scale cell culture applications. Due to the pre-competitive nature of the field, there is enormous opportunity for collaboration between academic researchers and industry partners, as well as for cross-applicable research that simultaneously would advance the technologies of other industries utilizing large-scale cell culture such as biologics, cell-based therapy, and regenerative medicine.
The companies currently pursuing clean meat production utilize a wide variety of approaches in terms of production processes and product focal area, and there is a crucial need for cross-collaboration between these companies and more established companies in the biomedical and life sciences. These relationships, in addition to the development of multidisciplinary consortia between academic researchers and industry partners, could bolster innovation and reduce duplicative effort in the clean meat field.
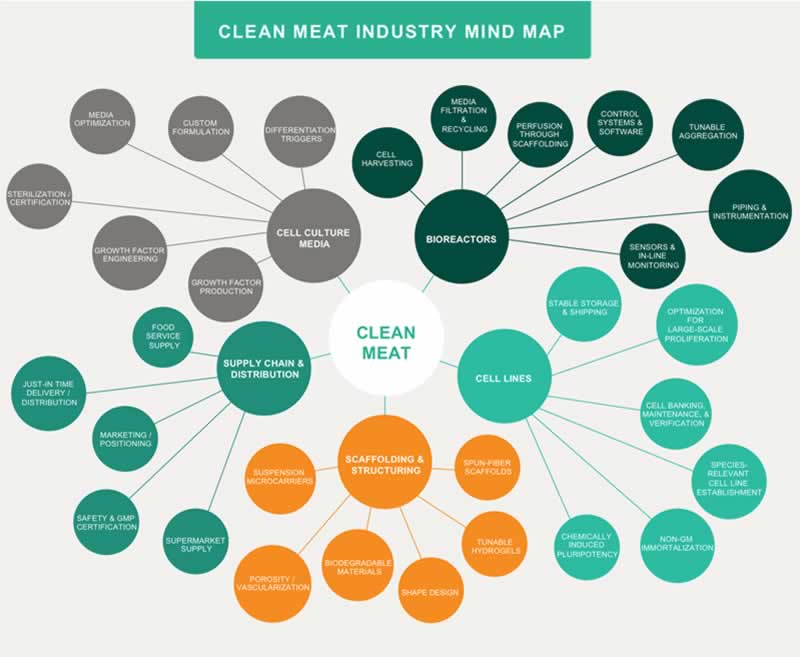
The primary challenge the clean meat industry faces is the scale-up and cost reduction of clean meat products. This endeavor can leverage insights and relevant technologies developed within established animal cell culture bioprocess industries. The largest commercial applications of animal cell culture are for protein therapeutics in the biopharmaceuticals industry, while the cell-based therapeutics industry is unique in producing cultured cells as the desired end product, rather than a purified protein component. The clean meat industry can also benefit from even larger scale bioprocess applications found in industrial biotechnology beyond animal cell cultivation; the production costs necessary to render clean meat economically viable will more closely mirror industries with large-scale fermentation for chemicals, food, and feed ingredients. Thus, a blending of techniques and insights from many bioprocess industries will benefit the scale-up process for clean meat.
Although many clean meat companies are in the early stages of product development and continue to evaluate a wide variety of production approaches, many of them will likely share the same basic process: large-scale cell proliferation in a bioreactor tank, followed by scaffold seeding and cell differentiation. The production timescale is dependent on several operating parameters including the degree to which the seed train is continuously run and, as a result, current production cycle estimates vary from anywhere between ten days and six weeks. Most estimates of theoretical yield in pounds of meat per 20,000 L batch range from 4,000 lb to over 10,000 lb. These may, in fact, be underestimates due to their reliance on parameters from CHO cells (for example, average cell volume) rather than tailored for the cell types that are relevant for meat.
Although the cultivation of cells for clean meat production is currently possible on a small scale, additional advances in cell line development and cell banking are needed to adapt cell cultivation for large-scale clean meat production. In order to reduce clean meat production costs, the development of robust cell lines that exhibit consistent performance over many generations and production cycles will be crucial. This will facilitate semi-continuous bioprocesses for cellular proliferation, limiting the need for batch processes for the final differentiation and maturation of cells. Utilizing well-characterized cells with rigorous cell banking protocols, in addition to semi-continuous seed trains, would also alleviate or eliminate the need for primary cell harvests that might introduce variability, potential contamination, and additional cost. To achieve this goal, footprint-free methods developed by the biologics industry to obtain cell line immortalization, methods for maintaining the stemness of pluripotent cells, and developments from the cell-based therapy industries regarding epigenetic influences and the induction of stable pluripotent stem cells should be considered. The use of novel techniques such as CRISPR and microfluidic screening may also represent a promising avenue for increasing performance attributes like the metabolic efficiency of cells. Attaining heightened cell efficiency, in combination with a decrease in the design requirements and other critical technological elements, will be instrumental in reducing clean meat production costs.
Building upon recent successes developing an array of serum-free and animal-origin-free media capable of supporting cell survival, proliferation, and differentiation, cell culture media customized for commercialized clean meat production will require consideration of a number of factors that are relevant within the context of a large-scale growth environment. These factors include parameters such as viscosity and pH in the fluid dynamics of large bioreactors, and stressors affecting cell survival that are exclusive to large-scale environments. Furthermore, in order to reach a price point competitive with conventional meat, large cost reductions of both the basal media and the growth factors will be required. Substantial cost reductions can be achieved simply through larger-scale production and increased tolerance for food-grade materials. Additionally, high-throughput screening can be used to identify formulation adjustments that will further reduce cost without significantly impacting cell performance.
Though they may not be strictly necessary for some first-to-market clean meat products which use cells as an ingredient for flavoring rather than for the entire product, tissue scaffolds that enable structural complexity will ultimately be essential to high-fidelity clean meat products. These edible or biodegradable scaffolds, which serve to support the co-cultures of multiple cell types and allow perfusion of cell culture media, may incorporate tunable parameters that can assist with temporally guiding cell differentiation. For example, hydrogel scaffolds have shown tremendous promise in other applications of tissue engineering because they enable precise and reversible modulation of scaffold biomechanical properties. Reversible control over biochemical parameters would offer clean meat scientists the ability to dynamically mimic the unique cellular demands that arise in an in-vivo environment as cells transition from proliferation to differentiation to maturation. However, additional research is needed to determine whether these materials are suitable for a food production context and if they are economically viable at the scales needed for clean meat. Scaffold development would further benefit from close collaboration with bioreactor developers to integrate scaffolds directly into the closed-system process design, which would significantly reduce the contamination risk of introducing a prefabricated scaffold.
As bioreactor technologies evolve to improve perfusion systems and increase maximum attainable cell densities, the breadth of published literature systematically comparing various bioreactor types and process design considerations will allow scientists to determine which designs are most suitable for clean meat production. The incorporation of additional parameters specific to clean meat production — such as sophisticated monitoring of culture medium composition and cellular phenotypic or metabolic traits — could benefit clean meat bioreactor design by enabling more nuanced control throughout the process. Biomanufacturing process automation will be critical to creating a functionally closed system that eliminates contamination risk, lessens variability from human handling, and reduces cost. Current large-scale harvesting systems developed for cell therapy applications are only suitable for single cells or aggregates, and pioneering efforts towards the creation of systems that enable the automated harvesting of large-scale intact tissues will be pivotal for the successful cultivation of clean meat products.
Due to the fertile nature of the clean meat field, there are significant opportunities for the development of strategic relationships between academic researchers and industry partners. There is a crucial need for open-access research, especially to investigate basic questions with significant downstream importance, and the development of academic/industry collaborative consortia could serve as an effective approach to ensure that early-stage research is performed with large-scale manufacturing considerations in mind. As modeled by other other disruptive technology sectors, robust dialogue between consortia members would de-risk investments by reducing duplicative effort and maximizing licensable opportunities for intellectual property developed by individual clean meat companies. These collaborative efforts could also prove informative for forecasting long-term hurdles and could be essential to the success of future developments such as the emergence of a clean meat-specific supply chain.
As the clean meat industry develops over time, research instrumental in the commercialization of clean meat will exhibit significant cross-applicability to other industries that utilize large-scale cell culture. As prototypes, demonstrations, and tastings of clean meat over the last two years have demonstrated, there are no fundamental technological impediments to the production of animal-free meat products. Rather, the pace at which collaborative relationships are fostered to address the primary challenge of increasing production process efficiency while decreasing costs will determine how quickly clean meat products gain traction in the consumer market.