Optimizing T cell media with animal component free, recombinant human serum albumin
Introduction
There are an increasing number of T cell-based therapeutics moving through clinical trials. These immunotherapies hold tremendous promise and much of the clinical trial data has been impressive. As these therapies have progressed toward larger trials and commercialization, focus is shifting from proof of concept to consistent and sustainable manufacturing.
In T Cell Therapy, a critical part of manufacturing requires that patients’ cells be expanded in culture to reach the desired dose. Thus, cell culture media is an important part of the process and must be able to both support cell growth and provide consistent results. In particular, there is a desire to move away from animal-derived and other undefined components in the media. These components can have lot-to-lot variability and increased risk of introducing adventitious agents. In addition, there is also concern around animal components and the effects accompanying cytokines/growth factors can have on cells.
Human serum albumin in cell culture
Human serum albumin, has a multifaceted biological role and its inclusion in cell culture media has been shown to be extremely beneficial for the propagation of many cell types ex vivo. Historically, serum has been used in most cell culture media either because cells needed it to survive or productivity was higher with serum. However, serum is a black box and researchers instead began to include only specific components of serum that were advantageous to cells. One of those proteins, albumin, is frequently added to media in place of serum. However, albumin isolated from human serum or bovine serum can still have variable performance and potential adventitious agent contamination risks.
Cellastim S – Recombinant human serum albumin
To address both the need for animal-component free media and the benefits that albumin provides in cell culture, InVitria has developed Cellastim S. Cellastim S is a recombinant human serum albumin that is completely animal component free. InVitria recognizes that the chemistry driving albumin performance in cell culture media is extremely complex and dependent on various modifications and ligands. As such, they have developed Cellastim S to specifically increase performance in animal component-free media for T cell culture.
To ensure performance, InVitria tests every lot of Cellastim S against multiple T cell donors This ensures Cellastim S performance, and minimizes the risks of lot-to-lot variability seen in FBS or serum-derived albumin products.
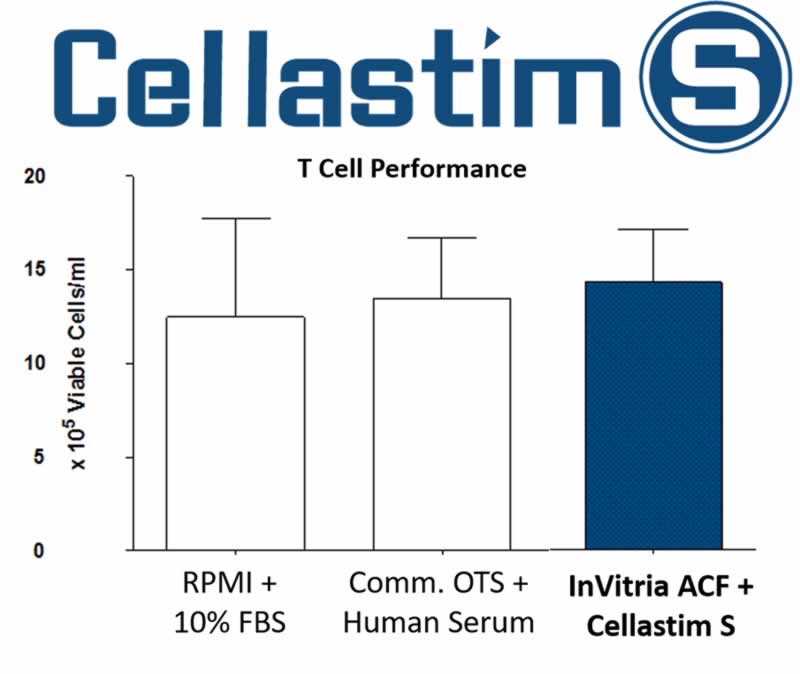
Figure 1 demonstrates that InVitria Animal Component Free (ACF) media is able to support equivalent T cell proliferation when compared to media supplemented with 10% FBS and Commercial Off-The-Shelf (Comm. OTS) media.
Figure 1:
For more information and a complete list of Cellastim S cell lines and applications, please see – Cellastim S or feel free to Ask an Expert.