Optimizing Virus Production Media for Cell-based Vaccine manufacturing
Cell-based Vaccine Manufacturing
There has been increasing interest in moving from egg-based vaccine manufacturing to cell culture-based methods. Cell-based vaccine manufacturing is quicker, efficiently scalable and offers more control over the manufacturing environment. There is also the advantage of fewer virus mutations and thus a more effective vaccine. This was certainly true with the 2017-2018 flu season. In a recent PBS article, “Flu vaccine grown without eggs provided measurably better protection this season, FDA says,” the FDA reported that the approved cell-culture based influenza vaccine performed about 20 percent better than the standard egg-based vaccines. This is due to the fact that the viruses have to adapt to grow in the eggs and with this adaptation can come mutations that render the vaccine less effective.
Vero Cells for Cell-based Vaccine Manufacturing
Vero cells have been very popular in the vaccine industry for over 3 decades. These cells have a long and successful history of safe vaccine production for many human and animal vaccines including polio, rotavirus, and smallpox.
Vero cells are adherent cells that require a surface to attach to in order to proliferate and produce viruses. Production scale-up with adherent cells is generally more challenging than with suspension cell cultures due to the surface area and media formulation requirements. Vero cells grow well in a variety of vessels including flasks, cell factories, and microcarriers in bioreactors, however they must be supported in attachment and need a robust media to ensure good growth and productivity.
Traditionally, Vero and other virus producing cells have been cultured in serum-containing medium. However, the vaccine industry has now moved toward serum-free media for Vero cell culture and vaccine production. Using media formulated without serum or animal components reduces risk of contamination with adventitious agents, streamlines regulatory documentation, and can increase consistency in culture.
Cell-based vaccine manufacturing media optimization
Current serum-free media formulations developed for Vero cell culture use high concentrations of plant-based hydrolysates to compensate for the removal of serum. However, the challenge with plant-based hydrolysates is that it is difficult to determine, with certainty, which exact components are providing which benefits to the cells. Thus, making it difficult to precisely optimize media components, attain chemical definition, and fully understand the best process for downstream purification. In addition, due to their undefined nature, plant-based hydrolysates can have variable performance.
At last week’s 2018 World Vaccine Congress in Washington DC, improvements to vaccine manufacturing were discussed. One of the areas of improvement was in chemically defined media for vaccine manufacturing. A poster, “Blood-free chemically defined virus production media for Vero cells,” was presented that provided a complete solution. In the poster, authors describe a study in which they were able to use a chemically defined virus production media (OptiVERO) containing recombinant human transferrin and recombinant human albumin to replace both serum and plant-based hydrolysates.
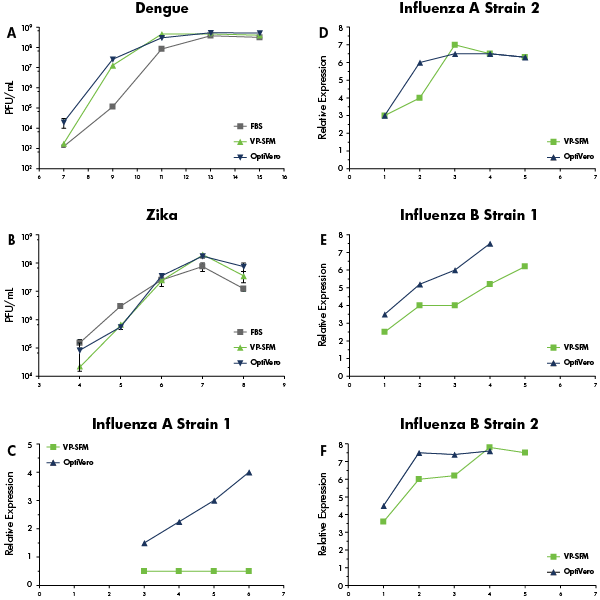
In the study, OptiVERO was compared to FBS and plant-based hydrolysates for VERO cell growth kinetics (Figure 1) and virus production (Figure 2). Based on the data presented, OptiVERO was able to support VERO in both 2D and 3D cultures. The new media also demonstrated equivalent production of flavivirus Dengue and Zika when compared to virus production serum-free medium and FBS. In influenza, OptiVERO demonstrated significantly better production capacity than that of virus production serum-free medium. This study demonstrates that Vero cells can thrive in a chemically defined media.
Figure 1: Growth performance in the blood-free chemically defined virus production media
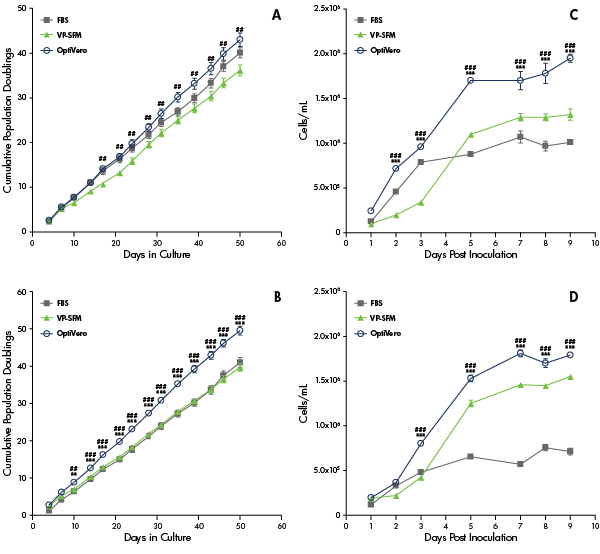
Figure 2: Virus productivity in the OptiVERO media are comparable to VP-SFM and FBS.