
Poster: Flow Electroporation Provides a Highly Efficient, Flexible and Scalable Transient Protein Expression System
As part of our Cell Line Development and Engineering Conference 2016 coverage, we will be writing about some of the posters presented at the conference. One poster that I thought provided a scalable and highly efficient system for transient protein expression was presented by MaxCyte, “Stable Antibody Production from CHO Cell Line of Choice Using Flow Electroporation.” In the poster, MaxCyte describes their flow electroporation transfection system that provides a cell type flexible and fully scalable method of transient gene expression.
Introduction
Transient transfection has been used to quickly and cost-effectively manufacture milligram quantities of recombinant protein in HEK 293 for years. The advantage of using transient transfection is that it takes significantly less time to generate material when compared with the alternative of developing a genetically stable cell line for manufacturing. This is particularly important in drug development where preclinical material is needed quickly in order to make informed go/no go decisions. Having access to preclinical material faster can greatly impact the overall drug development timeline. The challenge has been the transfection specifically of CHO cells to express the proteins of interest. CHO cells are typically more difficult to transfect, but are the preferred vehicle of antibody production due to their use in downstream production of clinical material.
The Advantages of Flow Electroporation for Transient Protein Expression
MaxCyte has developed an efficient and scalable transfection system based on electroporation. Electroporation involves the application of an electric field to cell suspensions, causing the cell membrane to become transiently permeable, which then encourages the entry of external materials into the cells. By utilizing the principles of electroporation, MaxCyte has developed a system that permits transfection of a wide range of cells with DNA, RNA, proteins or cell lysates and has demonstrated typical transfection efficiencies and cell viabilities at greater than 90% even for more difficult to transfect cells such as CHO cells. Another advantage of using electroporation is that it does not require specialized constructs, engineered cells, media additives or chemical reagents. The high performance of MaxCyte elctroporation produces CHO based protein titers at > 2.7 g/liter. This scalable system is able to transfect from 5×105 cells in seconds using small-scale, static electroporation to 2×1011 cells in less than 30 minutes by using flow electroporation.
MaxCyte’s System Details
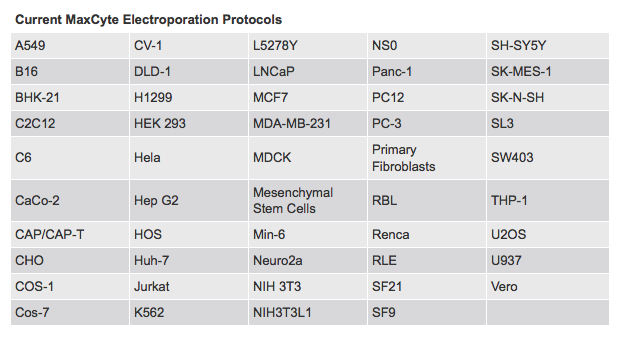
MaxCyte instruments are supplied with pre-loaded electroporation protocols optimized for a wide range of cell types (Figure 1), thereby simplifying assay development and maximizing performance and reproducibility. Identical electroporation parameters and cell handling are used for small and large-scale electroporation enabling streamlined scale up to high throughput transfection. MaxCyte transient transfection is completed in three steps: cell harvesting, electroporation, and post electroporation cell usage.
Figure 1:
Poster Highlights
In the poster, “Stable Antibody Production from CHO Cell Line of Choice Using Flow Electroporation,” MaxCyte describes their system and presents data to demonstrate the advantages of the flow electroporation approach.
Scalable
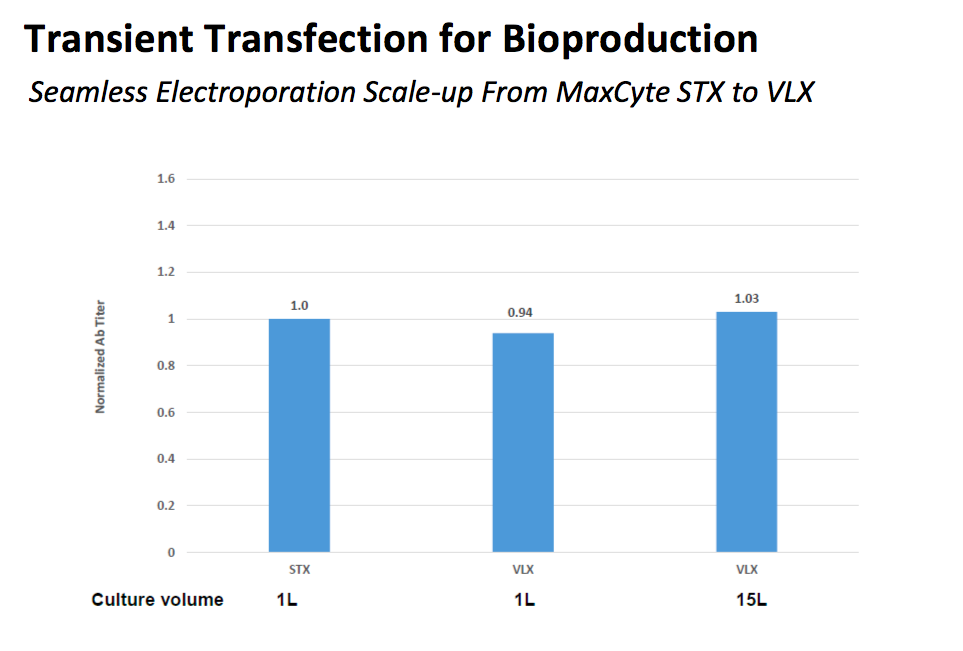
MaxCyte offers the MaxCyte STX and the MaxCyte VLX to address both small and larger scale production requirements. Scale up from the STX to the VLXrequires no further optimization. Results in the MaxCyte STX and the MaxCyte VLX are consistent thus offering a streamlined scale up solution (Figure 2).
Figure 2:
High Performance
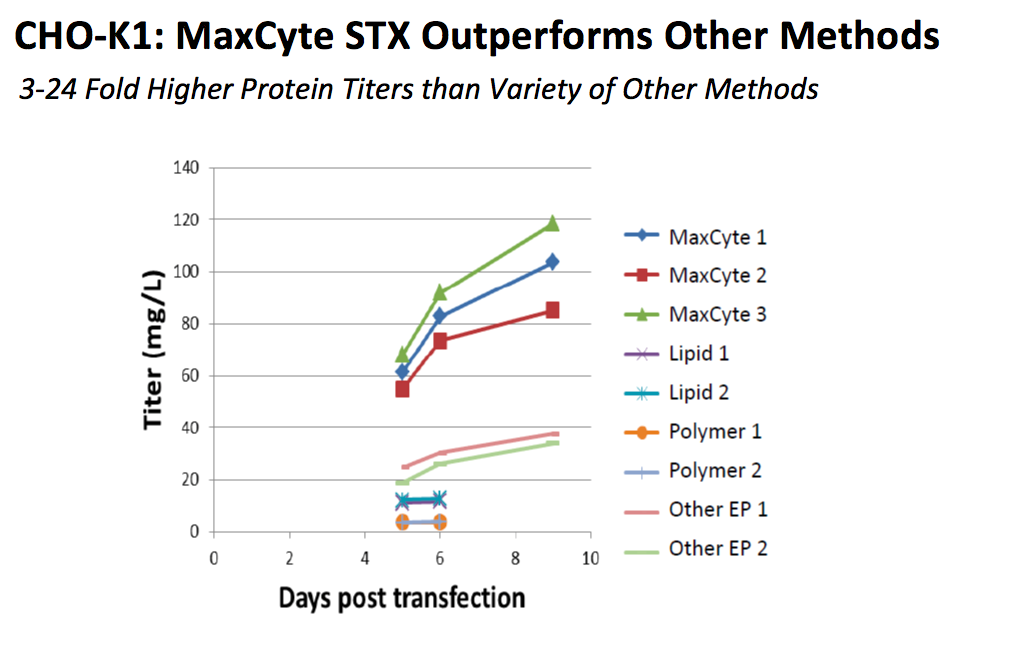
Using MaxCyte’s flow electroporation system, CHO cells can produce secreted antibody titers over 2.7 g/L with optimization of post transfection culture conditions. MaxCyte has presented data in the poster to demonstrate how the STX system compares to other methods of transfection in CHO K-1 cells (Figure 3).
Figure 3:
Flexible
A variety of CHO cell lines including CHO-S, CHO-K1, CHO EBNA, CHO-K1SV, and CHOZN can be transfected using MaxCyte transient transfection, resulting in higher titers than alternative transfection methods.
Figure 4:
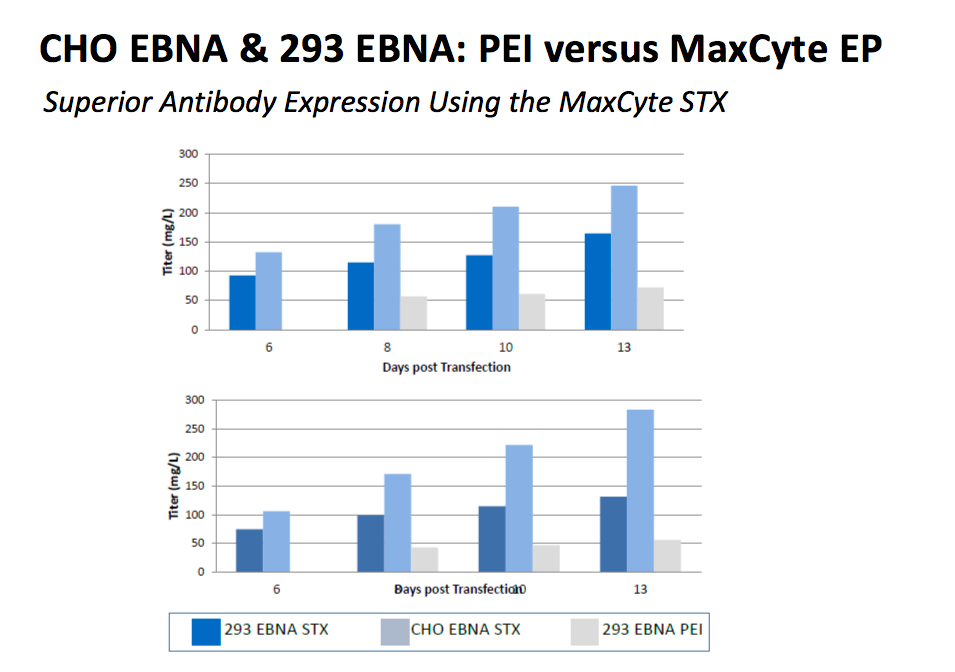
CHO EBNA and 293 EBNA cells were transfected with an IgG expression plasmid via static electroporation (6E7-8E7 cell per condition) and cultured in 125 mL shake flasks for 13 days. Secreted antibody titers in both STX-transfected cell lines greatly exceeded titers generated by an optimized PEI transfection method of 293 EBNA cells.
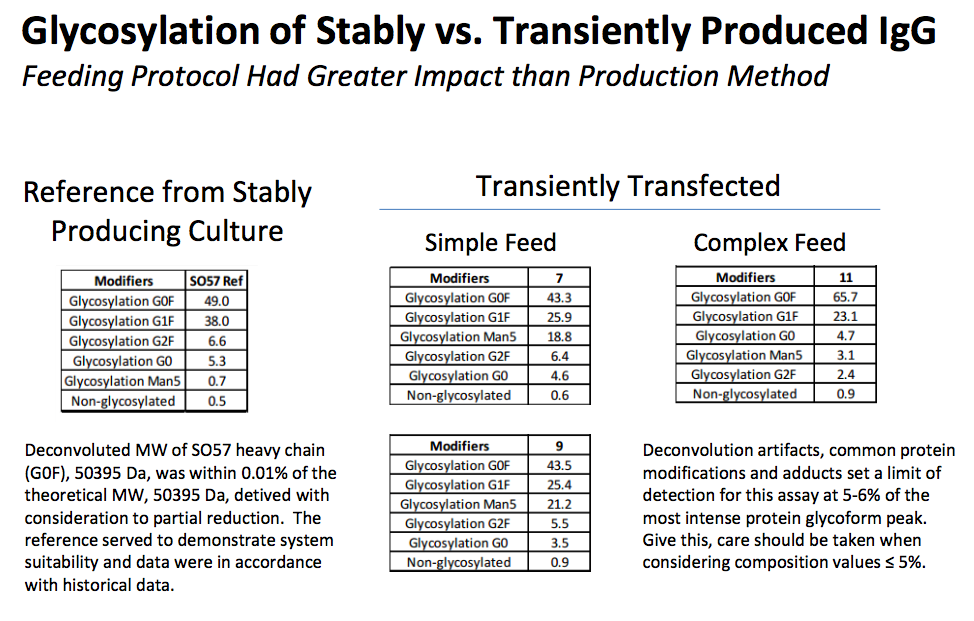
Equivalent Quality
MaxCyte transient transfection results in quality antibodies with glycan profiles similar to reference antibodies produced from stable production cultures. In Figure 5 the glycan profile was compared between stable culture produced antibodies and those produced using transient transfection. The results indicate that post transfection feeding had a more significant impact on glycan profiles than production method.
Figure 5:
For more data and full details of the highlights discussed here, please click on the poster below to view in full size.
