
Qualifying a rapid qPCR mycoplasma test approved for CAR-T clinical studies
Like other biologics, most cell therapy products require microbial testing including, mycoplasma, sterility, and endotoxin analysis as part of the final product release criteria. However, cell therapy products have unique QC product release requirements that necessitate new microbial testing technologies to support these unique needs. One of these is that since the cells are the final product so they cannot be put through a final sterile filtration step or undergo a harsh sterilization step and have a limited shelf life. As a result, it is a critical to implement rapid microbial testing for in-process and final product release that are compliant with the stringent regulatory requirements and also meets the unique QC needs for cell therapy products. Traditional testing methods for mycoplasma, sterility and endotoxin can take several weeks to over a month and require a large amount of sample volume which doesn’t fit the needs of limited sample availability and quick turnaround time for cell therapies. This is why rapid microbial methods, including rapid PCR mycoplasma methods, provide an important solution to a significant challenge in cell therapy quality management.
Rapid microbial methods are alternative methods that can detect microbial contamination faster than traditional testing methods and are easier to implement in-house. The quicker turnaround time from sampling to results supports final release testing closer to actual final product release and faster product release, thus enhancing the product’s safety profile and distribution speed. Additionally, many rapid microbial methods are able to be automated providing flexible throughput through clinical manufacturing. The quick turnaround time, lower sample volume requirements, and ability to automate also permit reliable, close to real time in-process testing, thereby enabling process analytics and quality by design initiatives. Stated benefits of rapid microbial testing include: significantly faster results, reduced product release times, reduced sample volume required and results more representative of final therapy product. Additional benefits realized from bringing rapid microbial testing in-house include: improved process control and cost savings.
There is a misconception however, that qualifying a rapid microbial method is challenging or that regulatory bodies are unwilling to accept this method. Thus I was encouraged to see Angeliki Grammenos, PhD, Celyad presentation at the 4th Annual CAR-T Congress in Boston. In the talk, Dr. Angeliki Grammenos shares Celyad’s experience in qualifying a rapid mycoplasma test that was approved for multiple clinical studies by multiple regulatory bodies. We are fortunate to be able to share a video of the presentation, “Using Roche MycoTool qPCR Assay for ATMP Release of CAR-T Cell Product Approved for Clinical Study by FDA, FAMPH, and MHRA,” here along with a summary of Dr. Angeliki Grammenos’ talk.
Celyad History
Dr. Angeliki Grammenos began the talk by describing Celyad’s background and extensive manufacturing experience in phase I, II & III in Cardiology & Immuno-Oncology (EU & US). Celyad has been working in cell therapies for 11 years and has become an expert in clinical manufacturing with over 100 employees in the US and Belgium. They have clinical trials in all phases of clinical development and most recently have focused on CAR-T therapies for treatment of relapsed refractory acute myeloid leukemia (rr AML) and the path to commercialization. They are working on autologous and on allogeneic CAR-T therapies and as Dr. Angeliki Grammenos shared, have been able to overcome the issue of immunogenicity. They are also working on developing treatments for solid tumors using CAR-T therapeutics.
CAR-T Therapeutic Manufacturing
She then went on to describe the Celyad manufacturing facility and process. Celyad has been able to reduce their autologous CAR-T therapeutic production time to 8-10 days including QA/QC with real-time release. She said that they were able to achieve this by critically looking at each step of the process and in-process testing instead of only testing at the end of the process. She shares data on transferring a CAR-T therapy from the Dana Farber Institute and how they were able to further optimize the process, which led to substantial log increase in cell yield vs. academic process. She explained the ability to increase cell yield was important to deliver multiple higher doses. Celyad’s current manufacturing success rate is 94% and their 1,100 square meter facility with two segregated manufacturing spaces in Mont-Saint Guibert is equipped to supply all of Celyad’s expected clinical trials and over 1,000 patients annually. Because they supply multiple products to multiple clinical sites in the US and the EU they are close to the airport and also successfully streamlined logistics to make this possible.
Characterization and Product Release
As the purpose of the talk was to focus on the qualification of a rapid nucleic acid amplification mycoplasma method, an alternative mycoplasma method, Dr. Angeliki Grammenos began discussing the release criteria for Advanced Therapy Medicinal Products (ATMP).
Microbiological safety testing is required for sterility, mycoplasma and endotoxin. The mycoplasma testing required per PhEUR 2.6.7 and USP <63> to release ATMP is mostly harmonized. With the culture method (Agar and Broth method) and indicator cells method as listed options. The limitations of these options are that they both have a long turnaround time with testing duration taking up to 21+7 days. Dr. Angeliki Grammenos pointed out that the patients are in advanced stages of disease and lengthy waiting for treatment just isn’t an option even for cryopreserved final products. In addition, with both of these options there is no possible way to automate the process, which means that it is not applicable for high-throughput and commercialization.
Qualifying a Rapid Mycoplasma Testing Option
When Celyad began searching for an accelerated mycoplasma release testing option years ago, FDA told Celyad to search for a vendor and to qualify the method chosen on their test samples in terms of sensitivity, specificity, and reproducibility. After discussing with regulators and researching, Dr. Angeliki Grammenos said Celyad discovered that not only was it feasible, it didn’t appear that it was going to be that difficult as both the PhEur and FDA “left the door open for qualifying an alternative method.”
Based on regulatory guidance, PhEur has these requirements for alternative rapid testing
- Nucleic Acid Amplification (NAT)
- Direct amplification is allowed if a rapid method is needed
- Amplification after enrichment (mostly to increase the sensitivity)
- Validated per monograph guidance
- Comparability study
- LOD at 10 CFU/mL for alternative to culture method
- LOD at 100 CFU/mL for alternative to indicator cells method
Roche Custom Biotech – MycoTOOL
After identifying vendors with rapid mycoplasma testing options, Celyad selected Roche Custom Biotech’s MycoTOOL. Dr. Angeliki Grammenos explained that they chose the MycoTOOL because it met all their requirements including the fact that it had been fully validated per PhEur 2.6.7 for specificity, detection limit and robustness.
Other Advantages included:
- Fast Read Out
- Nucleic Acid Amplification (NAT)
- Set up and validated on LightCycler System
- Fit for automation (from DNA extraction to PCR) – long term suitability
- Applicable on cellular matrices and non-cellular matrices (raw material release)
She then discussed key considerations for implementation. Because they were currently outsourcing their qPCR assay, the cost was comparable to traditional methods of routine testing in 4 wells/sample. She followed up by stating that there were such time benefits that it made the cost investment well worth it. She said that an alternative sampling strategy might be required depending on product volume/concentration. The extraction method is validated on MagNA Pure 24/96 system, which might be important to some end users.
Dr. Angeliki Grammenos then discussed the validation process. Because a full validation of MycoTOOL was performed by Roche per PhEur 2.6.7 with a detection limit from .01 CFU/mL to 10 CFU/mL this was sufficient to validate the test itself. She shared a quote from the PhEur 2.6.7 guidance that states that “When the analytical procedure is fully or partly implemented using commercial kits, aspects of validation already supported by the manufacturer, supporting documentation being available, can be omitted by the user.” This meant that Celyad did not need to cover all the requirements for validation the kit itself because Roche had already done this. However, the guidance goes on to say, “Nevertheless, this one must demonstrate the performances of the kit related to the intended use.” Simply put, Celyad had to show the kit was applicable to their product, intended use. This meant that they would have to show matrix inhibitory interface properties at 10 CFU/mL (LOD, specificity).
At this time they did have a slight problem with the concentration working in more than 10 billion cells even with dilution. They decided to conduct the test on the drug substance, before cryopreservation. This approach worked and the right sensitivity and concentration was achieved. They also felt that this was better scientifically as it was the actual drug substance they were testing. Regulatory authorities approved the use of their mycoplasma testing method on the drug substance.
Matrix inhibitory interference testing:
- 1 mL last culture supernatant (extracellular myco fraction)
- 106 cells (intracellular myco fraction)
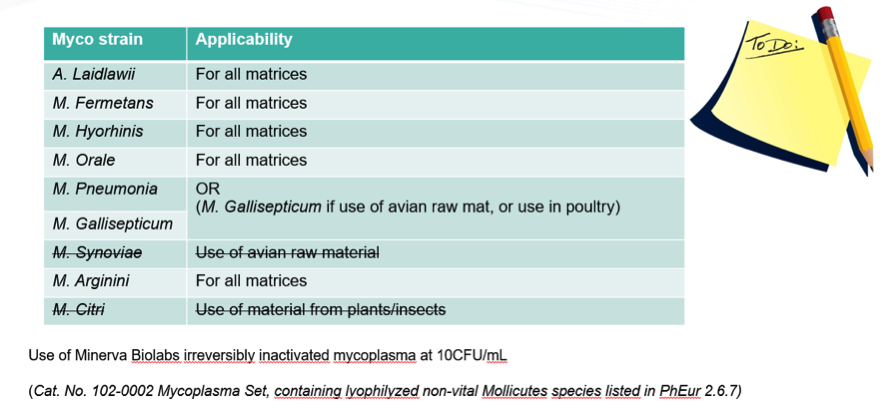
Dr. Angeliki Grammenos also explained that they worked with regulators to only test the mycoplasma strains according to Ph Eur 2.6.7 that were applicable to their product which didn’t include avian or insect/ plant related strains. She shared the standards Celyad used for matrix inhibition/ interference testing were irreversibly inactivated mycoplasma at 10CFU/mL from Minerva Biolabs. List of complete strains used and Minerva Biolabs part number are shown in the figure below.
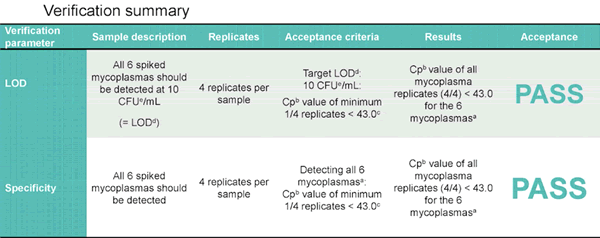
Final results demonstrated that the drug substance passed acceptance for both LOD and specificity with respect to mycoplasma release testing. Verification summary below:
Summary
In summary, Dr. Angeliki Grammenos stated that the MycoTOOL is suitable for rapid and sensitive testing of mycoplasma in cellular product (both drug substance and/or drug product). Celyad qualified this method for their CYAD-01+CYAD-101 ATMP drug products clinical studies with the United States, Belgium and United Kingdom authorities. Additionally, the matrix interference testing was easy to perform with inactivated and calibrated lyophilized mycoplasma. As a result of implementing the Roche CustomBiotech MycoTOOL Mycoplasma Real-Time PCR Kit, Celyad reduced testing time from twenty-eight days to three days for final product release and only need a single one milliliter of their drug substance for testing. Dr. Angeliki Grammenos shared cost is comparable to the traditional culture method since they are currently outsourcing testing, but said they end up gaining because of the time-savings. As Celyad has future plans to bring MycoTOOL mycoplasma testing in-house and this will lead to further reducing testing time to be same-day and significantly reduce cost.
MycoTOOL Mycoplasma Real-Time PCR and QC Sample Preparation kits are for use in quality control / manufacturing process only. All MagNA Pure 24 and MagNA Pure 96 kits, consumables, and accessories are for in vitro diagnostic use unless otherwise noted. The LightCycler® 480 system is for life science research use only. Not for use in diagnostic procedures.
MycoTOOL, MAGNA PURE, and LIGHTCYCLER are trademarks of Roche.
Other brands or product names are trademarks of their respective holders.