
Quality by Design – Maintaining Strict Control of Bioprocess Conditions
Introduction
Biomanufacturing processes are becoming increasingly complex, due in part to the increasing complexity of the biopharmaceuticals themselves and the need to decrease costs and speed time to market. To meet these demands, biomanufacturing of pharmaceuticals is increasingly being done with a Quality by Design (QbD) approach. A QbD approach provides a systematic look at bioprocess development, which attempts to predict unique challenges and address them in advance. To achieve this, critical process parameters must be identified and monitored closely throughout the process. However a difficulty in this approach is to ensure that the culture is being monitored precisely, so that critical process parameters can be maintained accurately.
For QbD to work effectively you must be able to maintain tight control of your bioprocess conditions and that can be achieved through reliable analytics, characterization of influencing factors, minimizing user interference and early detection of deviation. As a result there is a need for tools to achieve these goals. One important method is to collect regular samples and monitor the substrates and metabolites using a metabolite analyzer. This provides routine updates on how the culture is performing.
Achieving Strict Control of Process
One method for achieving tight control of a process and meeting QbD criteria was covered recently in a very informative webinar titled “Optimizing Control of Cell Culture and Microbial Fermentation in Bio-Manufacturing.” The webinar was presented by Dr. Christian Weilke, International Product Manager, Roche Custom Biotech and discussed Roche’s line of Cedex Bio Analyzers and how they can enable implementation of a Quality by Design (QbD) approach with the goal of attaining high quality, consistency and high yield in biomanufacturing.
The webinar began with a discussion on how to maintain critical process parameters once they have been identified. Maintaining culture in this optimal range for cell health, viability, productivity and product quality can be a challenge. To be successful it means that the culture must be monitored regularly to ensure substrates and metabolites are maintained within the identified range appropriate for that cell line and product. If substrates such as glucose and glutamine are too low then cells will be limited in growth and productivity. If metabolites such as ammonia or lactate are too high then growth and productivity are limited and corrective measures or early harvest are necessary to avoid product degradation. To maintain this fine balance, regular sampling and testing are necessary; if the testing method is imprecise then the corrections will either be too late or inaccurate.
Roche Custom Biotech’s Cedex Bioprocess Analyzers are designed to help identify and maintain this optimal range. The line is comprised of the Cedex Bio and Cedex Bio HT. These analyzers are able to provide valuable information about the health and productivity of cells while in culture. This offers the opportunity to make real-time adjustments and provides the potential to reduce cycle times and production costs.
Dr. Weilke then presented a case study in which an undisclosed biopharmaceutical company had a cell line requiring glucose to be maintained within a small, specific range. If the glucose concentration was too high there were glycosylation problems with the product, if it was too low the cells died. To achieve this narrow range, the company employed Roche’s Cedex Bio Analyzer to test the culture every 12 hours. If the glucose dropped below the target amount, additional glucose was added to reach the desired range of 2.95 – 3.0 grams per liter. When they compared two processes monitored with either the Cedex Bio or a membrane analyzer they found that with the membrane analyzer, the glucose testing was less precise, causing glucose levels to fluctuate more widely and often they were outside of the desired range.
After investigating the difference it was discovered that for the membrane analyzer, the measured concentration of glucose was not as precise, therefore it was often different than the actual concentration of the culture. This meant that glucose was often added when it wasn’t truly needed, it wasn’t added when it was needed, or the incorrect amount was added, see Figure 1.
Figure 1
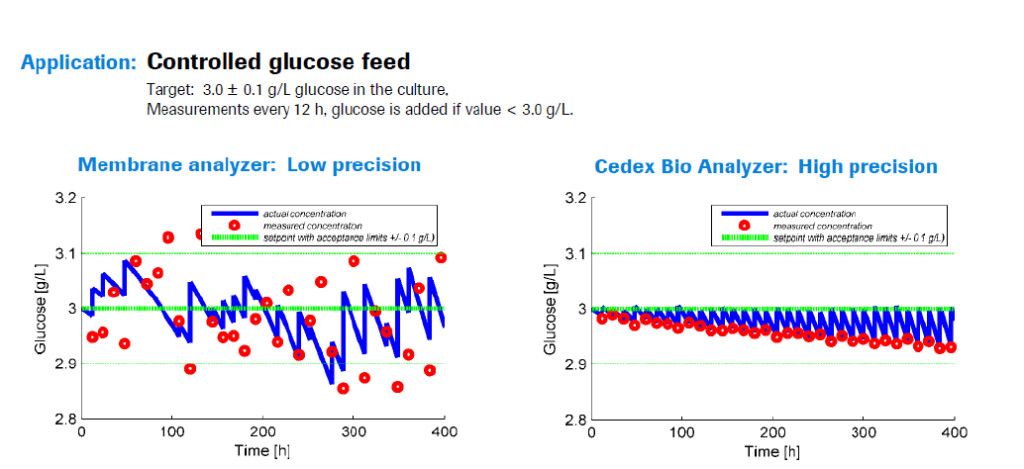
In order for QbD monitoring and control of culture to be successful there must be high reliability and equivalence to HPLC reference, something that was achieved with the Cedex Bio.
Dr. Weilke then discussed the benefits of implementing QbD and supporting with Cedex Bio Analyzers. These benefits included a shorter time to market and higher productivity of the culture, see Figure 2.
Figure 2
Dr. Weilke closed the webinar with an interesting look at how to create a completely closed loop, automated system leveraging Bayer Technology Services’ BaychroMAT product for sterile sampling, accuracy of Cedex systems, and live data transport via GU’s Sm@rt Line Data Cockpit software. By using a combination of products from three companies, they were able to show feasibility of a closed loop concept for bioprocess monitoring.
For more information on creating a closed loop for monitoring, please see this webinar or our previous blog, “The Benefits of Implementing Bioprocess Monitoring and Quality by Design in Process Development.”
About Cedex Bio Analyzers
The Cedex Bioprocess Analyzers employ the same technology used in the In-Vitro Diagnostic market. Roche has been a leader in the development of diagnostic tests and analyzers commonly employed in laboratories and hospitals. Roche Custom Biotech has utilized this extensive knowledge to create a line of analyzers for use in bioprocessing.
A comprehensive testing panel covers common assays, both for mammalian cell culture as well as for microbial fermentation. In addition there are unique parameters available, including LDH, IgG, Magnesium, Calcium, Iron and Total Protein. Additionally, Roche has demonstrated that high quality and high reproducibility can be achieved using a small sample size, which is critical in an integrated process where samples are collected regularly. Roche has a number of tests currently available on both the Cedex Bio and Cedex Bio HT for mammalian cell culture as well as bacterial or yeast production, including 13 substrates, 4 metabolites and 2 product assays. They are continually adding new tests with several additional assays planned for launch this year and in 2016.
Summary
Quality by Design initiatives will continue to evolve as new tools and technologies are developed to both aid in the identification of critical process attributes and in the maintenance of these attributes. For anyone interested in learning more about how to develop a robust bioprocess monitoring system or maintain control of an optimal range of identified process attributes, I would recommend these webinars as a guide of the benefits of this type of monitoring and also key tools and considerations for moving forward.
Bioprocess Monitoring:
The Future of Bioprocess Monitoring: How Greater Precision can Improve Quality and Yield
Achieving Strict Control of Process:
Optimizing Control of Cell Culture and Microbial Fermentation in Bio-Manufacturing