
Rapid Mycoplasma Testing for Biomanufacturing Applications
Increasingly the quality of raw materials used in cell culture media and supplements is being closely examined for any potential negative impact on cell culture or the final product quality. One area of concern is biomanufacturing contamination. While there are certainly many possible entry points for cell culture contamination, contaminated raw materials is high on the list. Of the possible contaminants, mycoplasma is often a big concern because it is fairly resilient in laboratory environments, resistant to most, if not all antibiotics, and also contamination in culture is not always obvious. Yet, mycoplasma contamination can have a devastating impact on biopharmaceutical development and production. As a result, having adequate mycoplasma monitoring in place is critical for minimizing the risk and ultimately impact of contamination.
A recent webinar, “Optimization of the Roche CustomBiotech MycoTOOL qPCR assay for the mycoplasma testing of peptones used in bioproduction applications,” presented a very advantageous approach by using a commercially available real-time PCR kit for screening for mycoplasma in raw materials. In the webinar, Stacy Holdread, Staff Scientist, BD Bioscience, discusses a rapid mycoplasma testing method that her team implemented to test peptone media supplements.
Background
Stacey began the webinar by discussing how BD Bioscience develops high performance peptones and supplements for use in bioproduction applications. Their products are used in the manufacture of over 140 biopharmaceuticals and they manufacture off the shelf and customer designed media as well as provide custom media formulation services.
What are peptones?
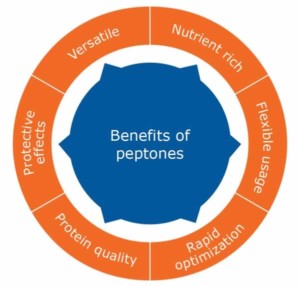
Stacy went on to explain that a peptone is a protein hydrolysate that is water-soluble. Peptones are created by partial hydrolysis of proteins from yeast, plant or animal sources. Peptones are widely used as media supplements because they offer numerous benefits to cell culture (Figure 1). Peptones can be incorporated throughout the development process in cloning, cell selection/expansion, media development and upstream process optimization. Prior to peptone product release, BD Bioscience conducts traditional and specialized release tests.
Positive contributions from a peptone
Mycoplasma Screening
One of the specialized release tests that BD Bioscience conducts is mycoplasma testing. Stacey explained that mycoplasma testing is important because it minimizes any risk of contamination downstream in bioproduction. She says that there has been an industry change in the understanding of how mycoplasma can be present in the media. Thus testing for mycoplasma contamination in the raw materials minimizes the possibility of mycoplasma entering the process and creating a more widespread contamination.
There are many possible sources for mycoplasma contamination including technicians, airborne particles and aerosols, incubators, liquid nitrogen, contaminated cell lines, media, sera, supplements, reagents, and improper sterilization. Because of the many possible entry points, it is important to screen at multiple points. Stacy shared a diagram of the possible screening points for mycoplasma (Figure 2).
Sources of mycoplasma contamination
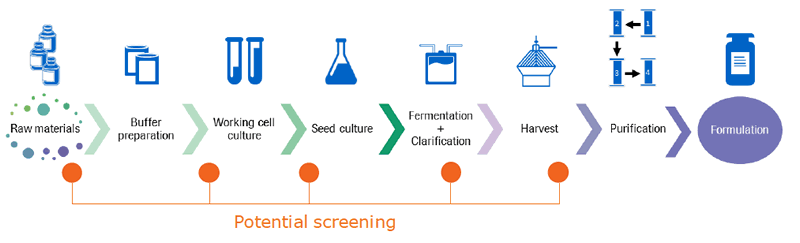
Stacy explained that managing high-risk raw material sources combined with rapid mycoplasma screening are key to minimizing risk of contamination. Early screening of the raw materials and in-process minimizes impact. Final lot release testing is required to meet regulatory requirements.
Next Stacy discusses how the biopharma industry has attempted eliminating mycoplasma contamination risk in media through various methods. Heat, radiation, pH shift, and antibiotics are all possible treatment methodshat have been used to try to eliminate mycoplasma, however mycoplasma has intracellular phases and thus is very difficult to eliminate allowing contamination to persist. Additionally, these treatment methods all can have negative impacts on the media performance and/or the cells themselves making them inadequate. Another possible solution is filtration, but this has its limits too in that mycoplasma can be very small and has no cell wall, thus it can change shape and under the right process conditions a filter can be ineffective. So the most powerful way to minimize risk is to screen materials prior to use.
Existing Mycoplasma Testing Methods
Advancement in mycoplasma testing technology has allowed the biopharma industry to get better at detecting mycoplasma contamination. Stacy shared BD Bioscience mycoplasma testing methods, benefits and shortcomings of each method, and their testing capability that relied on external testing laboratories prior to completing the new project (Table 1 and 2).
Primary mycoplasma testing methods
| Method | Overview | Benefit | Considerations |
|---|---|---|---|
Direct method
|
Test medium plated on agar and in broth. Broth subpassaged to agar during test After the required incubation period, the agar plates are observed microscopically for the presence of mycoplasma colonies. |
“Gold Standard” method Confirms if the cultures are live Long, successful track record of usage |
Long and labor intensive testing time Some strains are difficult to culture Trained microbiologist to read the agar plates Requires culturing live mycoplasma |
Indirect method
|
Test medium inoculated directly onto tissue culture cover slips or flasks containing a monolayer of indicator cells Cells are fixed and stained using a DNA binding stain (e.g. Hoechst stain) and contamination is confirmed by visual observation via fluorescent microscopy |
Confirms if cultures are live Detects M. hyorhinis Faster than direct method |
Labor intensive Skilled technician to run assay Less sensitive than direct method Requires specialized cells |
| Polymerase Chain Reaction (PCR) | Specific oligonucleotide primers capable of amplifying and detecting multiple species of mycoplasma DNA while excluding other contaminating DNA | Fast – results available in <5 hours Detects M. hyorhinis Accurate – uses primers specific for mycoplasma DNA |
Does not differentiate live vs. dead cultures |
Table 1: Mycoplasma testing methods
Current mycoplasma testing capability
| Test lab | Test method | Price per sample | Average testing time |
|---|---|---|---|
| Contract Lab 1 | PCR not validated | $150 | 10 days |
| Contract Lab 2 | Points to Consider (PTC) | $500 | 28 days |
| Contract Lab 3 | PCR validated | $2700 | 15 days |
Table 2: Mycoplasma testing capability
In discussing how their existing testing method was limited because they had to send all testing out to a contract lab, Stacy shared the primary limitations included:
- Sample submission only on certain days
- Each sample needed to be verified/validated prior to the first test, which added cost and time
- Long turn around time
- Limited availability to troubleshoot a testing issue
- Limited visibility and control over the testing
- Can be expensive
For these reasons, Stacy explained that they wanted the flexibility to be able to do mycoplasma testing in-house. The team began to explore in-house testing options and determine which technology would meet their needs for media and supplement testing. Their goal was to find ways to reduce risk and provide value to their customers via improved internal testing. The challenge was to identify a rapid and reliable mycoplasma testing technology that could be quickly implemented in R&D and RMS Pilot Operations and has met regulatory expectations for biopharmaceutical lot release testing.
After evaluating a number of options, BD Biosciences decided to move forward with Roche CustomBiotech’s MycoTOOL Real-Time PCR Kit. The MycoTOOL met their needs for high throughput with 96 well plates and with the capacity to go up to 384 well plates for even higher throughput. They also liked the kit design for the MycoTOOL and provided reliability and confidence in results. In the initial capability assessment, the MycoTOOL had all of the capabilities that they required for testing (Table 3).
Roche mycoTOOL capability assessment
| Requirement | MycoTOOL Capability | Met Requirement? |
|---|---|---|
| Reliable vendor with excellent technical support | Roche CustomBiotech support team | Long history of excellent support with Roche Cedex BioHT Analyzer |
| Broad usage | Accurately test cells, media, and peptones | Confirmation through internal testing |
| High sensitivity | Detection down to or below 10 CFU/mL.(1 CFU/ mL for most species listed in EP 2.6.7) | |
| High accuracy | Measures wide range of culturable and non-culturable species (> 150) | |
| High specificity | No cross reactivity with closely related bacterial species | |
| Reproducibility | Optimized kit controls and validated reagents | |
| Ease of use | Clear instructions, easy to use equipment | |
| Rapid | Results in < 1 day | |
| Clear results | Data reports provide easy to interpret data summary |
Table 3: Roche CustomBiotech MycoTOOL capability assessment
Roche CustomBiotech MycoTOOL Capability Assessment
Stacy and her team began their confirmation of the capability assessment through internal testing. They screened cell culture media and compared the results to the FDA Points to Consider method. Both the cell culture media and master cell bank testing of CHO and Vero cell lines using the MycoTOOL showed no contamination, which was previously confirmed with the FDA Points to Consider method.
For the initial peptone screen a variety of peptones were assessed. The results showed that 65% of the samples demonstrated a weak positive result for mycoplasma contamination. Representative samples were then sent to an outside testing lab to determine if they contained live mycoplasma. Testing confirmed that none of the peptones contained live mycoplasma and from this they determined that additional investigation was needed to adapt the method for testing of peptones. They worked with Roche CustomBiotech to modify MycoTOOL’s standard manual sample preparation protocol for peptone testing.
MycoTOOL Optimization for Peptone Testing
Several parameters were identified for optimization of peptone testing.
First, three different stock concentrations were tested at 10%, 1% and 0.1% solutions. At the 1% and 0.1% concentrations false positives were eliminated and background noise was minimized. They also examined sample enrichment at 5x, 10x and 20x, they found that low or no sample enrichment was ideal. The higher the sample enrichment, the more background noise. Next they optimized the number of PCR cycles by testing 48, 45, and 42. They determined that 42 was the best for reducing false positives.
After making the following parameter adjustments, they eliminated the false positive issue.
- 1% peptone solutions
- 0-5x enrichment
- 42 PCR cycles
- Template DNA volume should be determined for each peptone
- 1:10 dilution for template DNA.
Last they needed to confirm that the optimized peptone testing method did not negatively affect the ability of the MycoTOOL to detect a real mycoplasma contamination.
Mycoplasma detection studies were done where live mycoplasma cultures were inoculated into 1% peptone solution. Assay sensitivity was assessed using Mycoplasma orale, Mycoplasma arginine, and Mycoplasma hyorhinis.
They found that the method optimization resolved the testing issues with the peptones and changes to the method did not change the functioning accuracy or sensitivity of the assay. All three mycoplasma strains were detected in 1% and 10% peptone solutions down to or below 10 CFU/ mL.
After implementing these optimizations they determined that their method using MycoTOOL met all their needs (Table 4). Executing the testing was also very straightforward with test results in less than 5 hours. In addition, training employees to run the testing was simple and easily accomplished.
Roche CustomBiotech MycoTOOL capability Assessment
| Requirement | MycoTOOL capability | Met requirement? |
|---|---|---|
| Reliable vendor with excellent technical support | Roche CustomBiotech support team | ✅ |
| Broad usage | Accurately test cells, media, and peptones | ✅ |
| High sensitivity | Detection down to or below 10 CFU/mL. (1 CFU/ mL for most species listed in EP 2.6.7) | ✅ |
| High accuracy | Measures wide range of culturable and non-culturable species (>150) | ✅ |
| High specificity | No cross reactivity with closely related bacterial species | ✅ |
| Reproducibility | Optimized kit controls and validated reagents | ✅ |
| Ease of use | Clear instructions, easy to use equipment | ✅ |
| Rapid | Results in < 1 day | ✅ |
| Clear results | Data reports provide easy to interpret data summary | ✅ |
Table 4: Roche CustomBiotech MycoTOOL capability assessment results
Conclusions and Next Steps
Roche CustomBiotech’s MycoTOOL successfully met all requirements. As a result they are able to increase testing frequency and throughput, reduce testing times and testing costs (Table 5). They plan to also establish data packages for potential technology transfer to quality control. They were able to confirm that complex solutions like peptones can be accurately and reproducibly tested for mycoplasma, but that each product should be assessed to confirm method suitability. By optimizing the testing method using MycoTOOL they were able to resolve any issues around peptone testing.
Current mycoplasma testing overview
| Test lab | Test method | General price per sample | Average testing time |
|---|---|---|---|
| Contract lab 1 | PCR,Not validated | $150 | 10 days |
| Contract lab 2 | Direct method (PTC) | $500 | 28 days |
| Contract lab 3 | PCR Validated |
$2700 | 15 days |
| Internal | Optimized MycoTOOL method | $130 | <1 day |
Table 5: Mycoplasma testing cost and average time
Next steps are to continue assay validation work and expand use of the technology within the group.
Speaker Bio
Stacy Holdread, Staff Scientist, BD Life Sciences
Stacy Holdread is a Staff Scientist in the BD Biosciences Advanced Bioprocessing R&D group with almost 25 years of experience in mammalian and microbial media design and manufacture. Stacy worked on microbial media optimization and peptone development at Difco Laboratories for three years prior to joining the BD cell culture team. She has helped to establish BD’s mammalian cell culture media design program which is focused on the development of media, feeds, supplements, and processes for bioproduction applications. Stacy also played a key role in establishing BD’s scalable manufacturing process which encompasses product development, piloting, and GMP production. In addition to her product development responsibilities, Stacy continues to manage BDAB’s technical transfer process and provides technical manufacturing support. Stacy received her BS in Biology from the University of Michigan and her MS in Biotechnology from the Johns Hopkins University.
The MycoTOOL Mycoplasma Real-Time PCR Kit is for use in quality control/ manufacturing processes only.
MYCOTOOL is a trademark of Roche. Other brands or product names are trademarks of their respective holders.