Cool Tool – Rapid and robust AAV characterization
Adeno-associated virus (AAV) vectors are the most widely used gene delivery systems for new and established gene therapies due to their effectiveness at transferring beneficial genetic material into dividing and non-dividing cells with low pathogenicity and flexible tropism to target different tissue types. Because of their utilization in cell and gene therapeutics intended for patient use, governing regulatory bodies hold viral vector manufacturers to a high standard with the expectation that extensive product characterization is performed to ensure product safety and efficacy. The FDA’s chemistry, manufacturing and control (CMC) guidelines state that manufacturers must prove the identity, strength/potency, purity, safety and stability of their viral vectors.
AAV manufacturing challenges
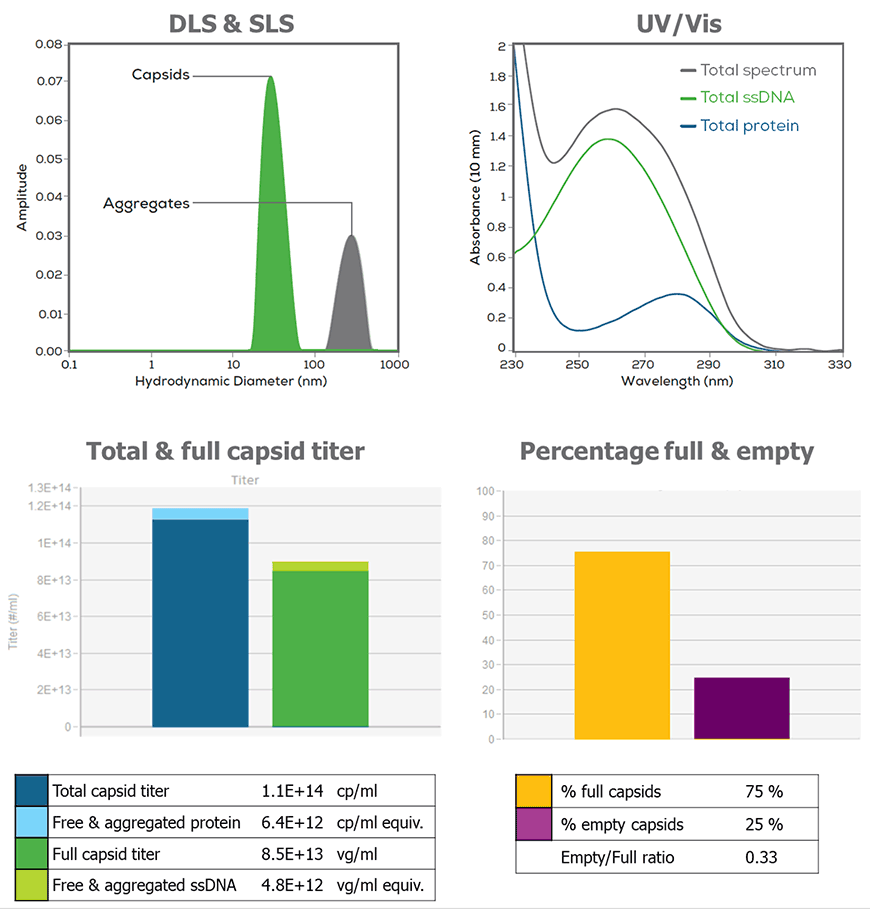
A major challenge in manufacturing AAV vectors is the concomitant formation of empty or partial capsids that do not contain the intended genetic sequence to exert a therapeutic effect. These unwanted vector-related impurities effectively reduce the potency, affect the purity of the vector product and may even confer a safety risk to the patient. Thus, AAV vectors must be fully characterized by comparing sequence-specific genome titers against a total capsid titer and the amount of total DNA present.
The need for better analytical tools
Analytical tools to accurately determine AAV capsid titer, capsid content (empty/full ratio), total DNA and protein present, and presence of AAV aggregation can help manufacturers better understand their vector product and optimize their processes to produce higher purity, more potent AAVs for therapeutic use. Current quantitative approaches include a combination of orthogonal techniques like analytical ultracentrifugation (AUC), transmission electron microscopy (TEM), PCR-based methods (i.e., qPCR and ddPCR) and colorimetric assays such as enzyme-linked immunosorbent assay (ELISA) to estimate AAV protein and genomic titer as well as capsid content. However, long experimental times, variable detection sensitivity/accuracy and destructive sampling greatly impacts their utility in process optimization and quality control analysis where near real-time results are needed. In particular, during in-process viral vector manufacturing optimization, limited sample volumes and detection of subtle changes in AAV parameters requires technologies that have the sensitivity, reproducibility and throughput to generate actionable data in a time-sensitive manner. These challenges highlight the need for better analytical tools in this space.

Stunner enables rapid and comprehensive AAV characterization
Stunner is one of those tools. It employs a unique approach to AAV characterization by leveraging UV/Vis spectroscopy (230-750 nm) combined with Dynamic Light Scattering (DLS) and Static Light Scattering (SLS) to enable rapid and comprehensive AAV characterization. The instrument performs UV/Vis spectral analysis from 230–750 nm and 0.03–275 OD that is used to accurately quantify proteins, DNA and RNA concentration of many common lab samples. For AAV samples, the ratio of protein and DNA present is related to the empty/full capsid ratio. However, the spectral data alone is insufficient to determine how much of that protein and DNA is packaged into AAV capsids – for that, a combination of light scattering techniques is used. Dynamic light scattering (DLS) measures the relative light intensity scattered by particles over time due to Brownian motion and provides information on the size, size distribution and aggregate status. DLS is commonly used in conjunction with static light scattering (SLS), which is based on Rayleigh light scattering principles where the light scatter intensity is directly proportional to the particle concentration.
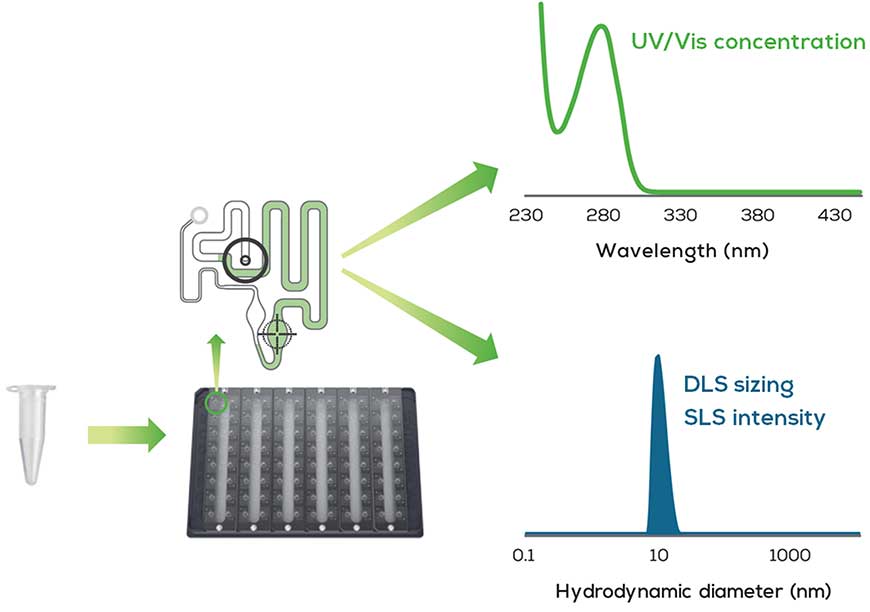
Combining DLS and SLS data together gives insight into particle concentration, but that answer still depends on whether the capsids scattering light are empty or full, since full capsids scatter more like than empty ones. When UV/Vis, DLS, and SLS datasets are combined, Stunner’s AAV Quant algorithm has enough information to calculate the vector genome titer, capsid titer, empty/full capsid ratio and aggregation status of any AAV sample, regardless of serotype.
The platform utilizes 96-well SBS-compatible plates. Each well is connected by microfluidic circuits to 2 microcuvettes with fixed pathlengths measuring 0.1 and 0.7 mm that capture sample absorbance precisely and across a wide dynamic range. Sample volumes of only 2 μL per well are needed for the analysis, which averages a run time of 1 hour / plate (96 samples). Stunner is also compatible with robotic automation platforms to enable even higher throughput and large capacity sampling requirements.
Stunner offers several advantages over PCR-based AAV quantification methods that use sequence-specific primers to target the AAV genome, which can miss DNA not captured by an appropriate primer. Stunner works across all AAV serotypes and different gene inserts without the need for primer optimization for each AAV. Capsid titer results generated by Stunner also correlate well with established techniques like ELISA where capsid titers for empty and full AAVs showed R2 values of > 0.98 and slopes near 1, demonstrating a high level of agreement between these two methods. But, because Stunner’s technology does not require standard curve generation or sample labeling, it requires less prep time than ELISA to bring accurate results to scientists faster.
Stunner meets the requirements for next generation analytical tools by executing rapid, comprehensive, low-volume AAV characterization at scale without costly sample preparation, calibration and technical resources required of other techniques. Thereby making it a perfect solution for early-stage, high-throughput screening. The platform’s performance meets USP and Ph. Eur. standards and is equipped with GLP-ready software for 21 CFR Part 11 compliance, which allows manufacturers the flexibility to utilize Stunner throughout all stages of development and production. It also eliminates the need for technology upgrades and re-validation procedures prior to commercialization. Planning for commercialization with the proper technology and procedures enables companies to progress at top speed to market.
For more information on Stunner, including access to application notes, webinars and demos, please visit: https://www.unchainedlabs.com/stunner/