Cool Tool: Rapid stability screening of AAV capsids and formulations
Vectors derived from adeno-associated viruses (AAV) are one of the most popular tools for gene therapies, immunotherapies and vaccines owing to their safety profile, efficient infection of dividing and non-dividing cells and stable maintenance of the viral genome without integrating into host cells. In the production of AAV vectors many variables can impact their stability, yield and, ultimately, their infectivity. Therefore, a detailed understanding of how factors such as the serotype, formulation, manufacturing method, and storage conditions impact AAV stability is essential for process development.
Notably, there are two AAV stability pathways that should be evaluated: capsid protein disruption and virus genome ejection from intact or partially disrupted capsids. Virus genome ejection can also be called uncoating. Thermal stability experiments can be used to measure AAV genome release by these two pathways. The Uncle platform from Unchained Labs, which is widely used for protein stability screening, has a Capsid Stability application that enables researchers to rapidly screen different conditions for their impact on AAV stability. Uncle is equipped with 3 detection methods: full-spectrum fluorescence, static light scattering (SLS), and dynamic light scattering (DLS) to provide orthogonal stability metrics from the same sample.
Uncle utilizes a thermal ramp to gradually heat samples to profile AAV thermal stability. Capsid protein disruption is measured directly from the fluorescence of the AAV protein capsid, while the addition of a fluorescent dye enables measurement of virus genome ejection. Beyond these two stability pathways, DLS quantifies sample size and size distribution prior to a thermal ramp, and SLS tracks exactly when aggregation begins during a thermal ramp.Uncle can measure up to 48 samples simultaneously and requires only 9 µL of sample in its multi-well quartz cuvette chambers, which can provide results in under 2 hours, much faster than traditional in vitro/in vivo assays (i.e., qPCR or infectivity assays).
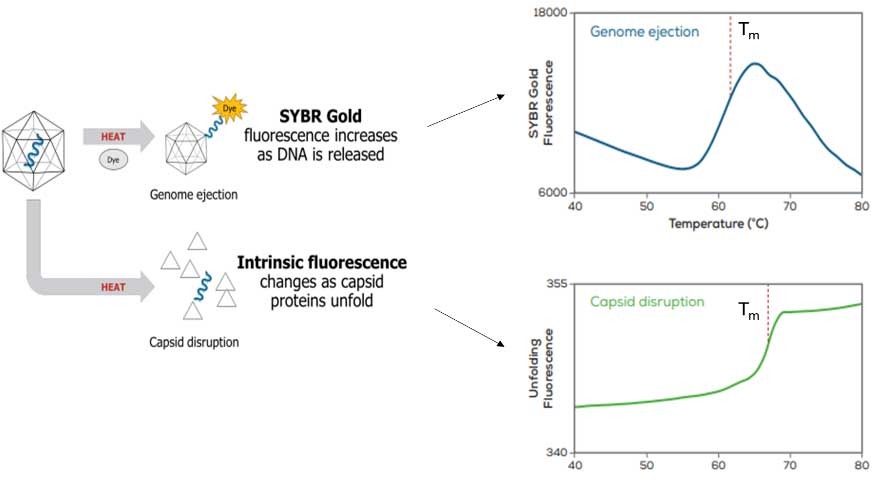
Figure 1 outlines how Uncle can be used to quantify AAV thermal stability based on DNA ejection or capsid disruption pathways. For virus genome ejection from intact capsids, Uncle can track changes in fluorescence intensity of DNA-binding fluorescent dyes, like SYBR Goldâ, where increases in fluorescence intensity represents DNA release from AAV capsids. Uncle can use the fluorescence data to determine the melting temperature (Tm) for each sample, which is the inflection point of the genome ejection curve. Alternatively, temperature-induced capsid protein disruption, where the viral capsid loses structural integrity as it is heated, can be studied by monitoring the changes in the intrinsic fluorescence of the amino acids in capsid proteins as they unfold, which can be used to determine the Tm.
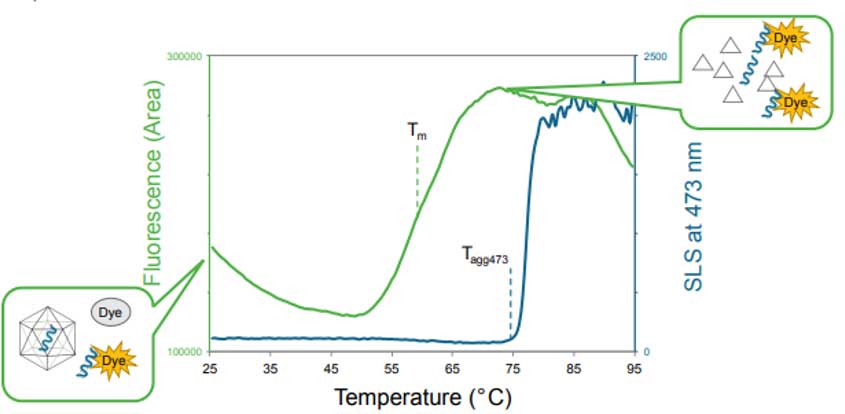
As part of the Capsid Stability application, the SLS signal of the 473 nm laser monitors AAV aggregation during the thermal ramp to provide information on the temperature at which particle aggregation begins (Figure 2). Additionally, DLS intensity can be used to determine particle size distribution, which is an orthogonal way to detect aggregation. The particle concentration and capsid content (empty vs. full capsids) can also be quantified by DLS.
With all this data gathered together, Uncle provides a convenient and rapid way to obtain multi-parameter data on the quality and stability of any AAV preparation and better understand the impact of factors such as serotype or buffer excipients. The ability to assess genome ejection, capsid protein unfolding, vector aggregation size and size distribution with one apparatus can streamline and accelerate workflows in the pursuit of novel gene therapies and the development of better vaccines.