Release Your Cells – A Simple New Tool that Eliminates the Need for Manual Selection and Scraping in hPSC Passaging
One of the things I enjoy most at a conference is learning about new technologies, especially those that remove or reduce obstacles in scientists’ workflow. One of the areas researchers frequently find challenging when passaging human pluripotent stem cells (hPSCs), is the manual selection and scraping technique used to remove differentiated cells. Articles protocol for passaging these cells requires that areas with differentiation be manually identified and removed, usually through scraping. The remaining hPSCs are then detached from the surface of the plate (by adding a passaging reagent), broken into aggregates and re-plated for further expansion. This method, while time-consuming, is still preferred by most over other hPSC passaging techniques due to:
- Proteases in enzymatic passaging reagents that digest cell surface proteins and can damage the cells.¹
- Disadvantages of simpler single cell hPSC passaging protocols, including: an increase in apoptosis and an accumulation of chromosomal abnormalities.²
Challenges with the Articles Protocol
When passaging hPSCs using the standard protocol, the first step is to identify and remove differentiated areas of the culture. This step relies on manual selection and scraping of differentiated areas and is dependent on an individual scientist’s skill. Thus, the efficiency of this step can be variable, and requires training and practice to acquire consistency. Despite experience, some differentiated cells may still be left behind and undifferentiated cells can be unintentionally removed. This aspect of hPSC passaging is laborious and not conducive to culture scale-up where closed vessels or other large culturing systems are used.
The next step is to add and incubate in a passaging reagent to detach colonies from the surface of the plate. Media is then added and colonies scraped off of the plate. Lastly, the colonies are broken up and the cell aggregates plated. Ideally aggregates should be 50-200 microns, so one needs to pipette up and down until the correct size is achieved. However, this process is again variable, as it requires a scientist to subjectively determine the appropriate aggregate size. The multiple steps involving manual manipulation are what make hPSC passaging such a labor-intensive technique.
A New Alternative
At the International Society of Stem Cell Research (ISSCR) Annual Meeting in June, I attended an Innovation Showcase presented by Dr. Erik Hadley that described a new solution to the pains involved in the standard hPSC passaging protocol. ReLeSR™, an enzyme-free passaging reagent launched by STEMCELL Technologies at ISSCR, enables consistent, high quality cultures by utilizing a simple protocol and a chemically defined formulation to eliminate the selection and scraping process.
The first thing that struck me when viewing the presentation was the difference in both number of steps and time spent when comparing the standard and the ReLeSR™ protocol (Figure 1).

How Does ReLeSR™ Work?
The ReLeSR™ protocol is so much simpler. You directly add and remove ReLeSR™ to the culture, incubating for 5-9 minutes, adding TeSR™ and tapping the dish. These three steps eliminate the identification and removal of differentiated areas and the breaking up of cell aggregates – the most challenging steps in the standard protocol process.
ReLeSR™ exploits the fact that differentiated cells are more strongly attached than undiffentiated cells. The undifferentiated cells then break apart and tapping the dish releases the cell aggregates and leaves the differentiated cells still attached. In addition, the resulting aggregates are the correct size without requiring additional breaking up of aggregates. The data shared at ISSCR also showed that there were fewer single cells and debris in the ReLeSR™ culture.
Quality of Cells
The obvious question is: what is the quality of cells generated with ReLeSR™? Data shared showed that the re-plated cells had the expected colony morphology and a similar purity and percentage of undifferentiated cells when compared with the standard method. Expansion seen was also comparable to standard enzyme-free protocols. Karyotyping of cells showed no evidence of abnormal karyotyping after 10 passages.
ReLeSR™ can even be used to rescue highly differentiated cultures. While it is not recommended to let your hPSC cultures have more than 20% differentiated cells, Dr. Hadley showed that adding ReLeSR™ to these cultures immediately improved and rehabilitated cultures to over 90% undifferentiated cells in just one passage. (Figure 2)
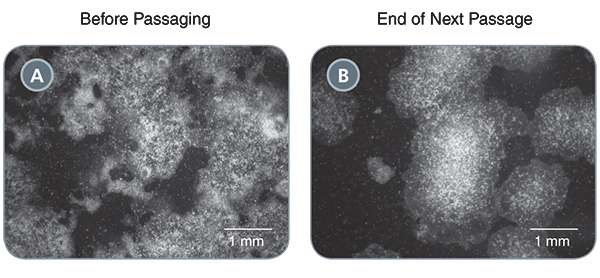
(A) A poor quality hPSC culture containing ~50% undifferentiated cells. (B) Following passaging with ReLeSR™, the differentiated cells have largely been eliminated from the culture, with >90% undifferentiated cells present at the end of the next passage.
Summary
Using ReLeSR™ to selectively detach differentiated cells removes the most labor-intensive and time-consuming aspects of passaging. In addition, removing the subjective steps of the protocol that rely on a scientist’s skill level and judgment can reduce the variability in culture. Lastly, by releasing the undifferentiated cells as mid-sized aggregates, it’s no longer necessary to manipulate the culture to generate cell aggregates at the appropriate size. ReLeSR™ is chemically defined and enzyme-free and is compatible with Vitronectin XF™ and Corning® Matrigel®.
ReLeSR™ fits nicely into STEMCELL Technologies’ product portfolio and serves as part of their complete workflow for reprogramming, maintenance, passaging and differentiation of hPSCs.
A recording of the Innovation Showcase on ReLeSR™, Scalable Enzyme-Free Protocols for the Isolation and Maintenance of Human Induced Pluripotent Stem Cells (hiPSCs) Without Mechanical Colony Scraping is available for viewing here.
References
- Beers J et al Nat Protocols 7 2029-2040, 2012
- Mitalipova et al. Nat Biotechnol. 23(1) 19-20, 2005