
Scalable AAV Production Platform from Bench to Commercial Manufacturing
Gene therapies treat disease at a molecular level by transmitting genetic information to a target cell to correct a genetic defect, to inhibit the expression defective gene, to restore normal function or to impart a new function to the cell to treat or prevent disease. The clinical successes of genetic medicines have driven increased investment to implement these therapies for broader indications beyond rare diseases. Adeno-associated virus (AAV) is the vector of choice of many in vivo gene therapies and as more therapies reach the clinic, technological challenges must still be overcome to fully unlock its potential.
A main stumbling block for the industry is successfully transitioning processes developed at bench-scale to commercial GMP manufacturing platforms to meet the growing dosage requirements for AAV. Fortunately, solutions providers have risen to meet the challenge by providing fit-for-purpose scalable platforms tailored specifically for viral vector production. Leveraging the know-how and technological capabilities of a single end-to-end solutions provider, such as Pall, can streamline the development of platform processes that can keep pace with the growing demand, with better productivity, process economies, and minimal risk.
Pall recently released a white paper entitled From Bench to Bedside: A Scalable End-to-End Solution for AAV Production, which highlights the benefits of a platformable and scalable end-to-end manufacturing solution for AAV. A feasibility study was conducted to evaluate the performance of a contiguous platform process to produce an AAV5 viral vector. Enabling technologies from Pall were used to create a single harmonized manufacturing solution aligned with good manufacturing practice (GMP) (Figure 1).
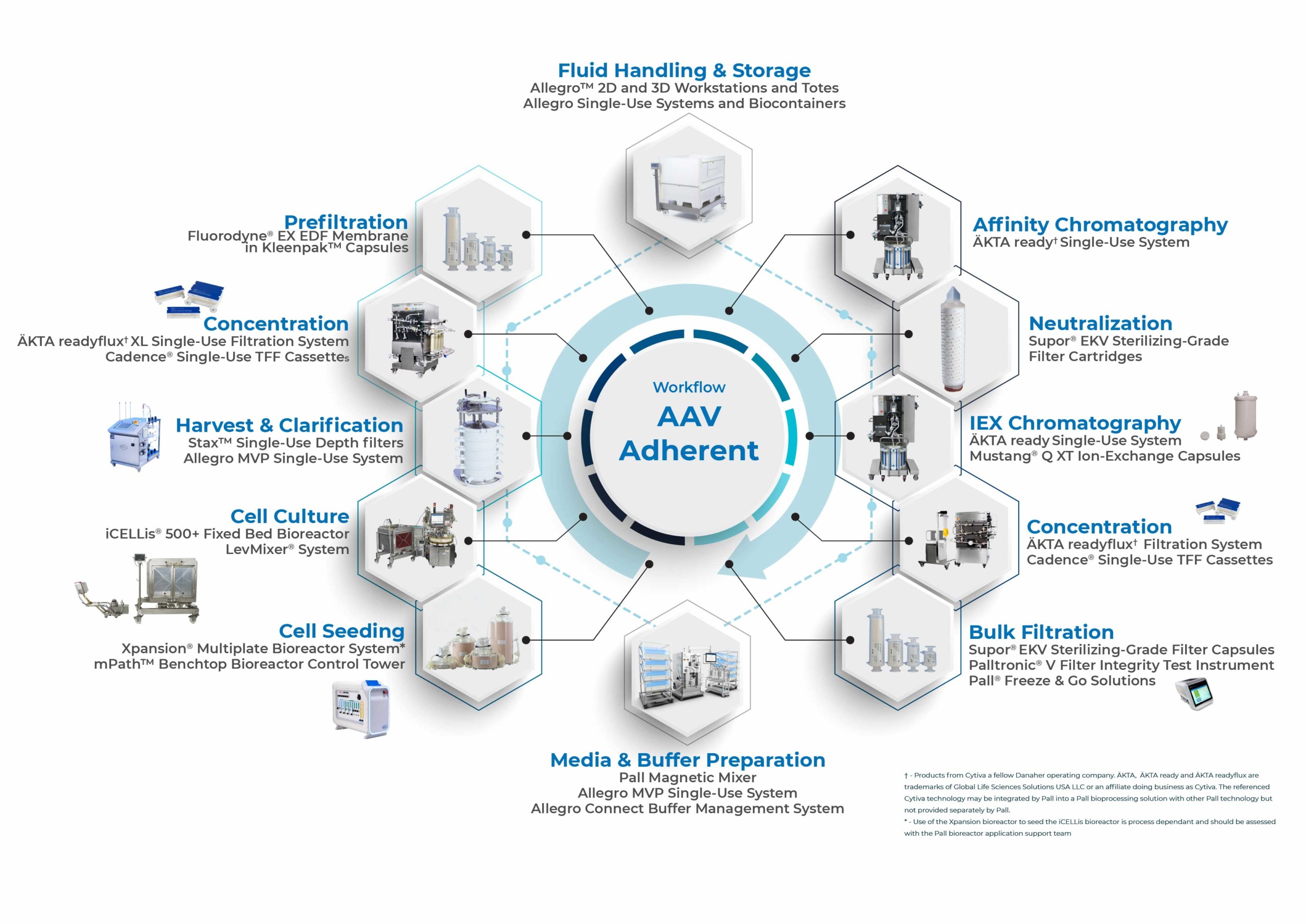
Below is a summary of the key manufacturing steps and results from the feasibility study outlining the effective implementation of Pall technologies for a representative AAV5 production process.
-
Viral Vector Production
Transient transfection of adherent HEK293 cells is one of the most widely adopted methods for AAV vector production. Pall’s iCELLis fixed-bed bioreactor system, with linearly scalable formats, can be effectively used to span requirements from small-scale early-stage (iCELLis Nano bioreactor) to late-stage clinical and commercial production volumes (iCELLis 500+ bioreactor). For the feasibility study, adherent HEK293 cells were expanded in the iCELLis Nano bioreactor until the cell density reached ~150,000 cells/cm2. Transient transfection and subsequent cell lysis produced a crude harvest with an average titer of 7.82 x 109 gc/mL.
-
Harvest/Clarification
Clarification of the cell culture harvest by filtration removes host cell impurities from the culture lysates to improve viral yield downstream. A filter train consisting of Pall’s P-grade PDK11 depth filter in series with a Supor EKV sterilizing-grade filter effectively reduced the crude harvest turbidity (from 57.9NTU to 2.9 NTU in clarified pool) with no observable loss in AAV step yield (104% as determined by ddPCR) in the AAV5 study. Given the known scalability performance of Pall’s filtration units, this direct filtration approach can be effectively implemented as a cost-effective and scalable clarification solution for large-scale AAV vector production.
-
Concentration
Pall’s Cadence® single-use modules with Omega 100 kDa PES membrane was used to concentrate the clarified AAV5 pool to a target volumetric concentration factor (VCF) of 10x prior to purification steps to reduce column loading/residence time. An average 64 LMH permeate flux of clarified pool with an average vector step yield of 91% was reported for feasibility study.
-
Purification (Affinity Chromatography)
Affinity chromatography is the preferred method for AAV selective capture where different affinity ligands are available for specific capture of various AAV serotypes.
-
Polishing by Membrane Chromatography
Anion exchange chromatography (AEX) polishing step typically follows affinity chromatography to further purify the feed before final formulation. For AAV, a big challenge is separating the full capsids that contain the genetic payload from closely related partial or empty capsids. These in-process contaminants need to be removed from the final drug product to ensure patient safety. Pall’s new step conductivity elution method using the Mustang Q membrane AEX chromatography can provide high resolution to effectively separate empty and full capsids. Across five separate feedstreams, an average of 5-fold enrichment of full capsids to total AAV5 capsids was achieved in the feasibility study.
-
Formulation
TFF is a scalable approach for ultrafiltration/diafiltration (UF/DF) to adjust the buffer and concentrate the AAV to the final formulation. The Cadence single-use module with Omega 100 kDa membrane was used for UF/DF of the purified AAV5 pool to achieve a target 10x VCF concentration and 7x diafiltration volume exchange into formulation buffer with high virus retention (≥99.9%) and final AAV5 yields averaging 89% over the four samples tested.
-
Sterile Filtration
Final sterile filtration is essential to mitigate microbial contamination of the final drug substance to ensure patient safety. Pall’s validated Supor EKV 0.2 µm sterilizing grade filters membrane filters in a range of volume formats (Mini Kleenpak 20 to the Nova capsules) are preferred for their low protein binding profiles for maximum transmission of the active ingredients. The AAV5 pool was sterile filtered with a Supor EKV filter in a Mini Kleenpak Syringe capsule (2.8 cm2) in constant flow mode with a flux target of 500 LMH. Virus concentration measurements the feed and filtrate pools showed high virus transmission (averaging 94%) through the sterilization membrane for maximum AAV5 recovery.
Overall, a holistic and integrated system that can support rapid scale-up, speed time to market, improve process economies, and streamline process will maximize the chance for overall project success. The feasibility study presented in the white paper emphasizes the value of a scalable end-to-end AAV manufacturing platform with technologies from a single provider. Beyond enabling technologies, Pall’s Accelerator℠ process development services are a resource that can guide developers through the transition from research to industrialized optimization of their specific gene therapy. A well-designed and cohesive platform process for an organization can serve to expedite the path to commercialization now and pay dividends in the future for emerging gene therapy modalities.
To download a copy of the white paper, please visit: From Bench to Bedside: A Scalable End-to-End Solution for AAV Production