
Scaling up Lentiviral Production from iCELLis® Nano Bioreactor to the iCELLis 500+ Bioreactor
The use of lentivirus vector in the production of CAR-T and gene therapies is increasing. Frequently, lentiviral vector production is conducted in multi-layered flatware vessels or fixed-bed bioreactors like the popular iCELLis bioreactor. The iCELLis bioreactor system is a fixed-bed bioreactor comprised of carriers made of non-woven medical-grade polyethylene terephthalate (PET) fibers. The iCELLis bioreactor provides optimal growing conditions for adherent cells employing a closed, automated, single-use platform.
The scalability of the iCELLis bioreactor was explored in a recent video and poster, “Scale Up of a Lentiviral Production Process from the iCELLis® Nano Bioreactor to the iCELLis 500+ Bioreactor,” published Cell & Gene Therapy Insights 2021; 7(9), 1023. Both video and poster describe an experiment conducted by Advanced BioScience Laboratories and Pall to scale up a lentivirus process from the iCELLis Nano bioreactor to the iCELLis 500+ bioreactor with very little development work. The goal was to produce lentiviral titers in the iCELLis 500+ bioreactor greater than or equal to the iCELLis Nano bioreactor and to use nutrient and metabolite analysis to determine if cell growth is similar between the two. Product was collected using perfusion after transfection. For methods and materials, please see the poster for details.
Results
The process was easily and efficiently scaled up from the iCELLis Nano bioreactor to the iCELLis 500+ bioreactor with an N=1 for each bioreactor.
Viral titer
The final bulk harvest had similar titer at three days post-harvest, suggesting that the concentration of virus in the perfusion out drum was the same as inside the iCELLis bioreactor vessel. Viral titer (gc/cm2) was 1.02 x 108 gc/cm2 in the iCELLis Nano bioreactor and 3.47 x 108 gc/cm2 in the iCELLis 500+ bioreactor.
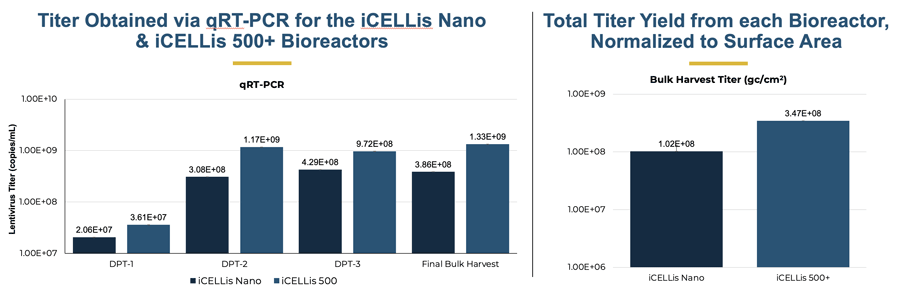
Left: Titer obtained via qRT-PCR for the iCELLis Nano and the iCELLis 500+ bioreactors. Samples were collected from the bioreactor vessels 1, 2, and 3 days post-transfection (DPT). Final bulk harvest was collected from the perfusion collection tote at the end of the run when the contents of the vessel were drained into it. Right: Total titer yield from each bioreactor, normalized to surface area.
Metabolites
The concentration of nutrients and metabolites were similar between the iCELLis Nano and the iCELLis 500+ bioreactors throughout the entire run, suggesting similar cell growth and density between the two scales.
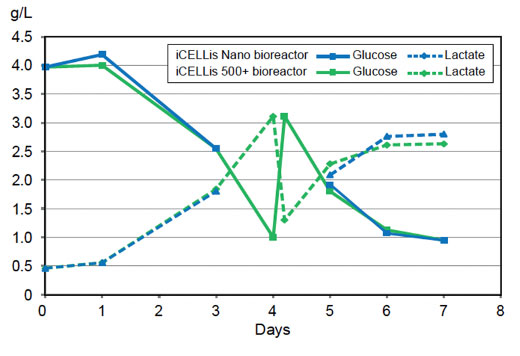
Nutrient and metabolite concentrations in the iCELLis Nano and iCELLis 500+ bioreactors in g/L.
Summary
The results of the experiment demonstrated that lentivirus production is easily scaled from the bench-scale iCELLis Nano bioreactor to the large-scale iCELLis 500+ bioreactor with very little development work required. This work supports the use of the iCELLis bioreactor across multiple scales for efficient vector production while permitting factors such as media volume per surface area, perfusion rate, fixed bed height, and fixed bed compaction to remain constant.
To view the video and download the poster, please see Case Study: ABL Scales up Lentiviral Production
About Advanced BioScience Laboratories, Inc.
(ABL) is a global contract development and manufacturing organization (CDMO) providing clinical supply solutions for vaccines, immunotherapies, oncolytic and genetic therapy agents, and other large molecule products. They have invested in the iCELLis bioreactor technology and are now demonstrating their use of the technology for lentivirus production at the typical production scale for CAR-T applications. ABL’s goal is to help customers reach the market quickly and efficiently with the iCELLis bioreactor.