
Single-Use Virus Filtration: Controllers and Systems for Modern Bioprocessing
Virus filtration is one of the last and most important safety steps in downstream bioprocessing. It removes potential viral contaminants and protects the safety of biologic products like monoclonal antibodies and recombinant proteins. In the past, stainless-steel, fixed-piping systems were the norm for this stage of manufacturing. As production environments have changed to emphasize flexibility, speed, and sustainability, single-use technologies have quickly become the preferred choice.
Single-use virus filtration systems remove the need for cleaning and validation between batches, which are two of the most time- and resource-consuming steps in traditional stainless-steel setups. They also enable a more flexible manufacturing approach, allowing for quicker turnaround times, easier transitions between scales, and lower risks of cross-contamination. These advantages make single-use solutions especially valuable for contract development and manufacturing organizations (CDMOs) and multiproduct facilities that often switch between products.
Asahi Kasei Bioprocess (AKB) has led this change by offering two complementary platforms that provide single-use performance in virus filtration: the VANTIJ® Single-Use Virus Filtration Controller (SU-VFC) and the VANTIJ® Single-Use Virus Filtration System (SU-VFS). These platforms aim to improve efficiency, sustainability, and compliance with regulations, representing a new way to ensure viral safety in biologics manufacturing.
The Single-Use Virus Filtration Controller (SU-VFC)
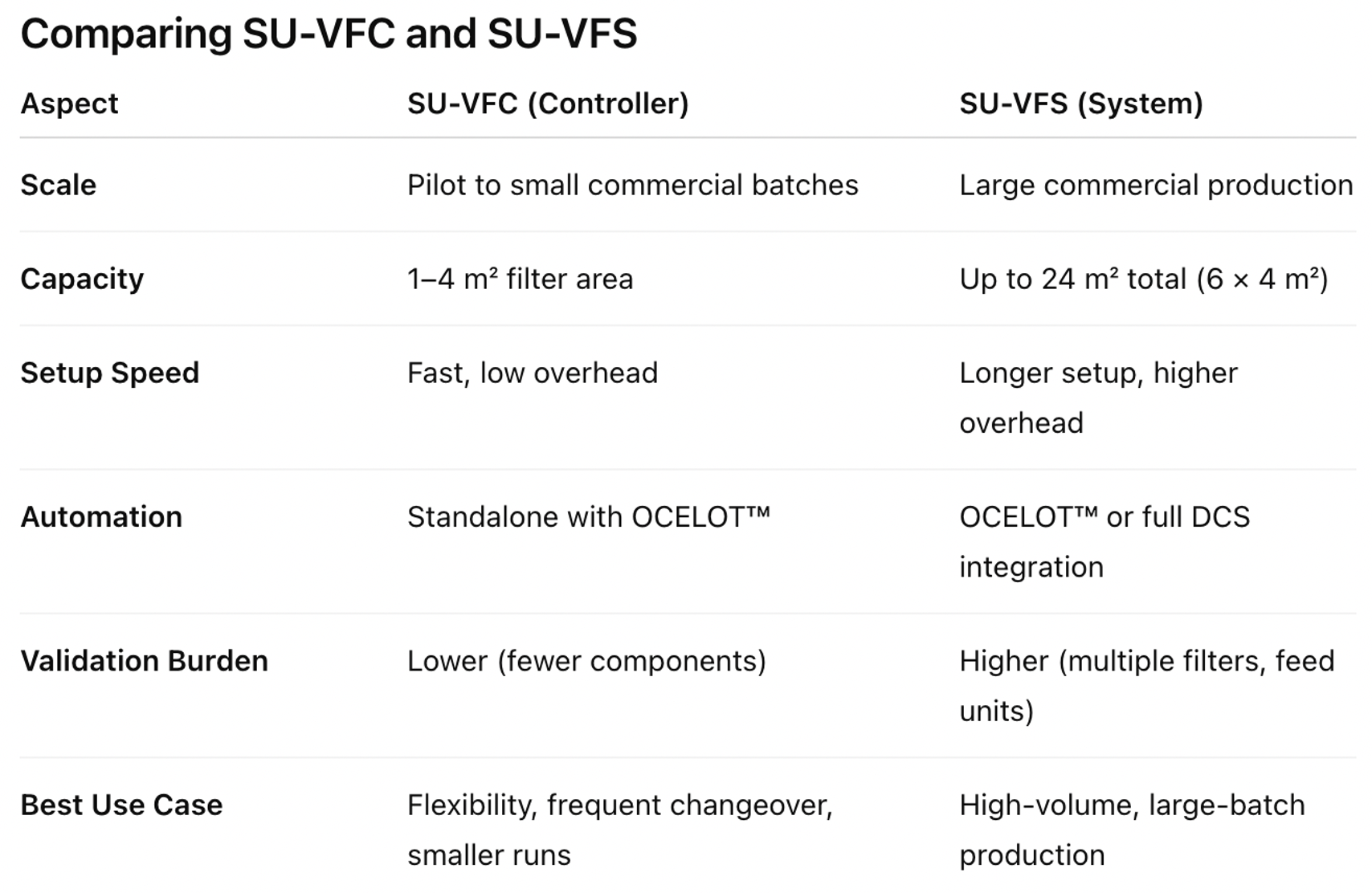
The SU-VFC was created to meet the growing need for an easy-to-use, compact, and highly efficient solution for small to mid-scale bioprocessing. By eliminating cleaning and sterilization, the SU-VFC boosts efficiency and productivity, allowing for new filtration runs to start without waiting for equipment turnover. This not only saves time but also cuts down on the use of water, steam, and chemicals, helping facilities strive for sustainability goals and reduce their environmental impact.
The controller works with AKB’s established Planova™ filters, including the Planova N series, BioEX series, and S20N. It supports both 1.0 m² and 4.0 m² filter modules. With flow rates from 20 to 1200 liters per hour at operating pressures up to 4 barg, it provides flexibility for both pilot-scale and small commercial applications. Despite its strong performance, the SU-VFC remains compact, allowing for installation in small cleanroom layouts.
A key feature of the SU-VFC is its automated system. Powered by the OCELOT® control system, it offers process control that is simple to use and meets 21 CFR Part 11 requirements for electronic records and audit trails. Operators benefit from easy integration with other equipment and distributed control systems (DCS), which support modular and connected manufacturing environments.
Operationally, the SU-VFC automates crucial steps in the virus filtration process, including pressure calibration, priming, integrity testing, flushing, filtration, and post-use verification. These automated features ensure consistency, traceability, and repeatability while reducing the chances of operator error.
The system comes with single-use tubing sets that are gamma-irradiated and preassembled, making deployment straightforward. This design allows for immediate use, significantly cutting down setup and validation time compared to traditional systems. The combination of swift readiness, automation, and minimal manual handling enables facilities to increase throughput and operate with fewer disruptions.
The Single-Use Virus Filtration System (SU-VFS)
The SU-VFS builds on the same design ideas as the SU-VFC and extends these benefits to large-scale commercial manufacturing. While the SU-VFC is tailored for small and mid-scale operations, the SU-VFS is designed for scalability, supporting up to six Planova™ filters of 4.0 m² each, providing a total effective area of 24 m². This capacity allows for high-throughput virus filtration in commercial production while maintaining the ease of use and reliability seen in the SU-VFC.
The SU-VFS includes customizable tubing sets, pre-filter configurations, conductivity monitoring, and visual leakage testing to match specific process needs. Its feed unit modules, , manage buffer, wash, and integrity test solutions, ensuring precise control over every stage of the filtration process.
Once again, automation is a key component of this system. Facilities can use the validated OCELOT® control for the SU-VFS or integrate it within their own DCS for consolidated automation. This seamless connection allows virus filtration to fit well into larger automated process workflows, helping manufacturers work toward continuous and modular bioprocessing.
The design of the SU-VFS prioritizes sustainability and flexibility across production scales. By removing the need for cleaning and sterilization, the system greatly cuts down on water and resource use. For organizations focused on environmental, social, and governance (ESG) efforts, this efficiency directly supports sustainability goals. The ability to quickly configure the system for various filter sizes or product types is particularly appealing for contract manufacturers serving multiple clients.
From a regulatory and validation perspective, AKB offers strong support for SU-VFS users. Viral clearance studies follow ICH Q5A guidelines, showing that target log reduction values (LRVs) are met. Customers also have access to extractables and leachables (E&L) testing, material compatibility assessments, and built-in pre- and post-use filter integrity testing. AK-Bio’s Technical Client Services (TCS) team provides customized training and ongoing consultation, acting as an extension of the customer’s own development team to ensure effective implementation and compliance.
Advantages of Single-Use Virus Filtration
AKB’s single-use virus filtration platforms offer benefits beyond convenience. They greatly improve efficiency and productivity since there is no need to clean or requalify the system between batches. They also improve sustainability by reducing the use of cleaning agents, water, and energy. Facilities gain greater operational flexibility, allowing them to switch between products or scales without the downtime of traditional stainless-steel systems.
Automation through OCELOT provides precision and repeatability. It ensures traceable data capture and meets regulatory requirements. By integrating with other process systems, manufacturers can create efficient, modular production setups that support both batch and continuous manufacturing strategies.
Considerations
Validation remains a key requirement. As systems scale up, ensuring consistent performance across multiple filters and feed units needs strong qualification and documentation. However, AK-Bio’s integrated integrity testing, alignment with ICH Q5A guidelines, and thorough TCS support make this process easier for end users.
Conclusion
As biologics production shifts towards faster, more flexible, and more sustainable models, the demand for flexible virus filtration systems has increased. The VANTIJ SU-VFC and SU-VFS platforms represent this change. The SU-VFC provides agility and immediate readiness for small-scale and multiproduct operations, while the SU-VFS scales those benefits for commercial manufacturing with better automation, sustainability, and validation support.
Together, they show that single-use virus filtration is no longer just an alternative to stainless-steel systems. It is a key driver of next-generation bioprocessing. By combining effective automation, regulatory readiness, and design flexibility, these systems help manufacturers improve efficiency, meet global compliance standards, and prepare their production capabilities for the future.
To learn more, please visit Single-Use Virus Filtration System | VANTIJ®.