Small Molecules in Stem Cell Research
A Guest Blog by: Simon Hilcove, PhD, Product Manager, STEMCELL Technologies
Introduction
Stem Cell Research offers extraordinary potential to the future of regenerative medicine. Stem cells have the potential to:
- Form the basis of cellular therapies for diseases involving organ systems with limited regenerative capacity
- Provide additional systems for drug screening and toxicity testing
- Give insight into early human development and (in the case of induced pluripotent stem (iPS) cells) obviate the need for human embryos
All of these applications require efficient, reproducible and cost-effective methods for the generation, maintenance and differentiation of stem cells. Small molecules in Stem Cell Research can be useful to help us understand and regulate stem cell biology.
Although their use in Stem Cell Research is quite recent, small molecules have long been associated with biological discoveries. Our understanding of biological processes often develops from discovering or designing ways to perturb a given process and observing the subsequent effects. While genetic and cytokine/protein-based approaches have been widely used for this purpose, small molecules offer some distinct advantages:
- Can be cell permeable and have the ability to affect signaling pathways within the cell
- Effects can be finely tuned by varying concentration
- High purity and low lot-to-lot variability in activity
- More defined, stable and cost-effective than growth factors
Target-based screening
Small molecules with known mechanisms of action and/or molecular targets can be used in the place of their respective pathway ligands. For example, the GSK3 inhibitor CHIR99021 can replace WNT in a number of applications. Similarly, using small molecules that interrupt or enhance a particular biological process can help to understand the importance of a specific signaling pathway or the role of a protein within this process. For example, molecules such as CHIR99021 and PD0325901 have been used to stimulate self-renewal of embryonic stem (ES) cells and iPS cells, demonstrating the roles of GSK3/WNT and MEK/ERK signaling in the maintenance of pluripotency. (1-3) In another example, activation of the WNT pathway with CHIR99021 followed by WNT pathway inhibition with compounds such as IWP-2 was shown to induce cardiomyocyte differentiation in pluripotent stem cells, thereby demonstrating the importance of biphasic WNT signaling in cardiomyocyte differentiation. (4) These studies illustrate how small molecules can be as effective at regulating stem cell fate decisions as more expensive growth factors, and can be used to gain further insight into the molecular pathways involved.
Target-based approaches have also been used to probe and improve the reprogramming process for making iPS cells. Histone deacetylase (HDAC) inhibitors (e.g. Sodium Butyrate, Trichostatin A, Valproic Acid), histone methylation inhibitors (e.g. 3-Deazaneplanocin A, BIX01294) and DNA methyltransferase inhibitors (eg. 5-Azacytidine, RG108) all have enhancing effects on reprogramming efficiency, demonstrating the importance of an open chromatin state during reprogramming. The use of these chromatin modifiers, along with small molecules that promote stem cell self-renewal and survival, has vastly improved reprogramming efficiency. For example, the combination of PD0325901, Thiazovivin and SB431542, has been used to increase the efficiency of reprogramming human fibroblasts to iPS cells. (5) More recently, small molecules (CHIR99021, Forskolin, Tranylcypromine, Valproic Acid, and 3-Deazaneplanocin A) have been utilized to reprogram mouse fibroblasts without the use of genetic factors, (6) providing encouraging news for the future of cell-based therapies.
High-throughput screening
Small molecules are routinely used in high-throughput screening in the pharmaceutical industry as a means for unbiased biological discovery. Recent technological advances are making these approaches accessible to academic laboratories, especially as many universities develop their own screening facilities. The spread of this approach has led to the discovery of novel molecules capable of regulating stem cell fate. Moreover, the identification of the targets and pathways that these molecules affect has increased our understanding of stem cell biology.
Several challenges to pluripotent cell culture and utilization have been addressed through the high-throughput screening approach. Human pluripotent stem cells have low single cell viability, which makes genome-editing and cloning techniques difficult. Compounds such as Y-27632 (7) and Thiazovivin (8) were identified in high-throughput screens and have been shown to increase survival of single cells through inhibition of RHO/ROCK signaling. Screening for molecules that promote reprogramming in the absence of one or more canonical reprogramming factors led to the discovery of RepSox, (9) a TGF-β inhibitor.
Regulators of lineage-specific differentiation are critical for the realization of regenerative therapies, and high throughput screening methods have identified small molecules such as Purmorphamine, a Hedgehog pathway activator that promotes differentiation of mesenchymal stem cells; (10-11) IDE1 and IDE2, which induce definitive endodermal differentiation of mouse and human pluripotent stem cells; (12) and Cardiogenol C, found to induce cardiomyocyte differentiation of mouse ES cells. (13)
Conclusion
It is an exciting time in the field of Stem Cell Research as new biological discoveries are being made with tremendous potential for regenerative medicine. Small molecules are becoming an indispensable tool for the field, both in the discovery process and in the development of efficient, defined, and cost-effective protocols.
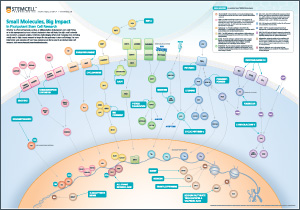
 For a FREE wallchart illustrating the small molecules and pathways involved in pluripotent Stem Cell Research, please click here.
For a FREE wallchart illustrating the small molecules and pathways involved in pluripotent Stem Cell Research, please click here.
References
- Gafni O, et al. Nature 504(7479): 282–6, 2013
- Li W, et al. Cell Stem Cell 4(1): 16–9, 2009
- Takashima Y, et al. Cell 158(6): 1254-1269, 2014
- Lian X, et al. Nat Protoc 8(1): 162–75, 2013
- Lin T, et al. Nat Methods 6(11): 805–8, 2009
- Hou P, et al. Science 341(6146): 651–4, 2013
- Watanabe K, et al. Nat Biotechnol 25(6): 681–6, 2007
- Xu Y, et al. Proc Natl Acad Sci U S A 107(18): 8129–34, 2010
- Ichida JK, et al. Cell Stem Cell 5(5): 491–503, 2009
- Wu X, et al. J Am Chem Soc 124(49): 14520–1, 2002
- Wu X, et al. Chem Biol 11(9): 1229–38, 2004
- Borowiak M, et al. Cell Stem Cell 4(4): 348–58, 2009
- Wu X, et al. J Am Chem Soc 126(6): 1590–1, 2004