
Solving Bioprocessing Bottlenecks with the HaLCon Analyzer for Real-Time Titer Measurements

This article was originally published in the eBook
Monoclonal Antibody Manufacturing Trends, Challenges, and Analytical Solutions to Eliminate Bioprocessing Bottlenecks. You can download all the articles in the series, by downloading the eBook.

Biologics, including monoclonal antibodies (mAbs) and therapeutic proteins, have had a profound impact on the therapeutic landscape by offering treatment solutions for a wide range of diseases. Their importance will continue to escalate in the years ahead, driven by the emergence of new targeted and personalized therapies. To meet the demand, ongoing innovation within the biomanufacturing sector is essential, with a focus on enhancing productivity and cost-efficiency to ensure broader patient access to these therapeutics.
In response to this, regulatory agencies have encouraged biomanufacturers to embrace the concepts of Quality by Design (QbD) and Process Analytical Technology (PAT)1 for process optimization efforts, such as upstream bioprocess intensification and continuous bioprocessing. This systematic approach focuses on the identification and control of critical process parameters (CPPs) that influence the critical quality attributes (CQAs) aimed at increasing process knowledge to enhance productivity and achieve high product titers while maintaining quality standards.
Successful PAT relies on the analysis and monitoring of CQAs within the bioprocess, such as protein titer. Protein titer serves as both a benchmark for yield estimation and a quality control metric, helping to guide the production process from early cell line development, process development, through to manufacturing. However, measuring protein titer in a bioprocessing environment has been a persistent bottleneck, especially when using instruments originally designed for research in a production setting.
PAT initiatives have paved the way for a new generation of analytical technologies specifically designed to provide timely and actionable protein titer data in bioproduction environments. New analytical platforms, such as RedShiftBio’s HaLCon Protein Analyzer fills a critical technology gap, providing researchers with the means to overcome industry bottlenecks head-on.
Challenges of HPLC in Protein Titer Measurement for Bioprocessing
High-Performance Liquid Chromatography (HPLC) with Protein A affinity capture is one of the most widely used methods for protein titer measurement and is considered the gold standard for protein quantification. However, its application in a production environment is hindered by the size and complexity of HPLC instruments. This makes HPLC accessibility at-line rare, limiting opportunities for real-time monitoring of product yields and the ability to implement real-time corrective action during a bioreactor run.
Typically, HPLC samples are processed in centralized Quality Control (QC) laboratories situated apart from the production suite. These core labs are often fielding measurement requests from multiple departments on multiple quality attributes, which can lead to HPLC backlogs. Furthermore, the time-intensive sample preparation process, the specialized training for HPLC operation, and instrument maintenance pose additional challenges.
This delay in time-to-data can holdup downstream purification processes that require protein titer information, such as scaling Protein A capacity, column selection, or determining the number of columns and cycles for simulated moving bed systems. Relying on retrospective data that can take days to generate is not conducive to the needs of bioproduction workflows, so alternative, automated methods are increasingly being considered as a replacement for HPLC.
Fit for Purpose Instrumentation
RedShiftBio’s HaLCon Protein Analyzer (Figure 1) is a compact and easy-to-use protein A liquid chromatography system purpose-built to meet the demands of the bioproduction environment2-4. Measuring less than 10 inches in width, the small footprint of the device allows it to be conveniently placed in a laboratory setting where bench space is at a premium or directly within a production suite, facilitating real-time data acquisition without disruption to existing equipment and workflows.
The instrument can be used at-line for protein titer measurement, with sample-to-data turnaround in under 5 minutes or integrated with an online automated sampler for seamless and hands-free sample analysis. Samples require minimal manipulation and can be prepared by filtering them through a 0.2 μm or 0.45 μm filter or by centrifuging and subsequently sampling the supernatant using a syringe. Operating the instrument is a simple process, involving just four steps: entering sample information, loading the sample, initiating the analysis, and swiftly obtaining results.
The plug-and-play system requires minimal (if any) maintenance and is designed with efficiency and user convenience in mind, consisting of two consumable parts, the reagent pack and the analysis module. This simplifies routine analysis by providing up to 1,000 samples with 90-day on-board reagent stability for each set of consumables. Optimized analytical conditions and pre-formulated reagents eliminate the need for method development, reagent preparation, specialized chromatography expertise and extensive operator training. This not only saves valuable time, but it also helps minimize user error to ensure consistent results. The sample flow path is fully enclosed within the analysis module, aligning with the requirements of single-use bioprocessing.

Chromatography Without Complexity
The HaLCon uses a proprietary analysis module containing a Protein A affinity column to quantify the antibody titer in a bioreactor over a dynamic range of 0.1–10 g/L in a cell-free sample with minimal sample manipulation and no dilutions required.
Users can actively monitor titer and acquire accurate at-line protein titer measurements, which correlate well with off-line HPLC and other protein measurement techniques like bio-layer interferometry (BLI). The instrument effectively eliminates bottlenecks associated with HPLC setup time, training, and the need to send samples to a core lab for testing, providing a more streamlined and efficient solution for titer measurement in the bioproduction environment.
A series of experiments designed to evaluate different performance aspects of the HaLCon Analyzer are outlined below.
Assay Linearity
In one study, the analyzer’s assay linearity and accuracy were compared with a Protein A HPLC system (Agilent 1100 HPLC system) across a measurement range of 0.1 to 6.5 g/L2. Figure 2 shows a calibration curve generated using a human IgG isotype control standard at concentrations of 0.1, 1.0, 2.5, and 5.0 g/L. The results demonstrate a wide dynamic range with an upper limit of 10g/L and robust linearity, with a linear regression (R2) value of 0.99973 within the specified measurement range.
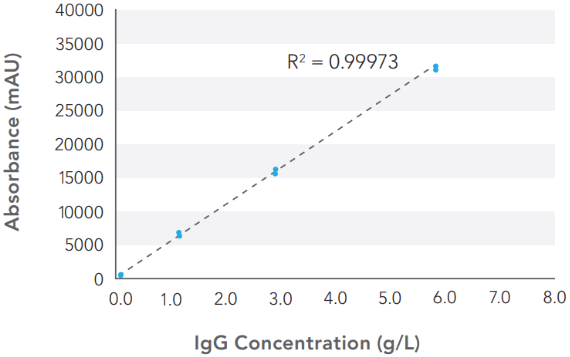
The extended dynamic range allows for extrapolation of values to higher titers, which is especially useful since current fed batch bioprocesses can yield protein titers >10 g/L.
The HaLCon can store multiple standard curves. This provides users with the flexibility to generate a specific standard curve using the antibody of interest or to select a more general calibrant for standard curve generation for use with multiple antibody products. Additionally, the standard curve remains valid for the entirety of the analysis module’s operational life, which spans 3 months or 1,000 samples (whichever comes first). This greatly streamlines the workflow and enables rapid sample analysis, while retaining high accuracy and precision for reported antibody concentrations.
Instrument Accuracy
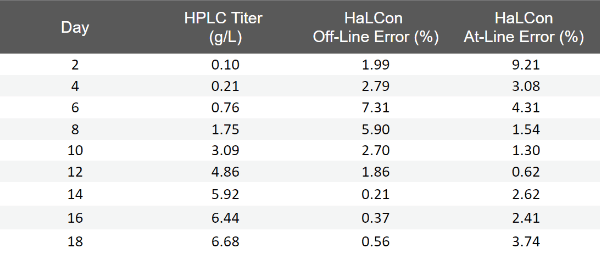
Manual and automated aseptic sampling measurements of a bioreactor run performed with the HaLCon demonstrated strong correlation with the off-line HPLC system. The automated aseptic at-line sampling was performed using the Flownamics Seg-Flow® 1200 with the F-Series FISP probe. Both the HaLCon Analyzer at-line and on-line titer values fell within 10% of the off-line HPLC titer values. Additionally, excellent intra-assay precision was evident, with a coefficient of variation (CV) below 3% observed for both off-line and at-line titer measurements (Table 1).
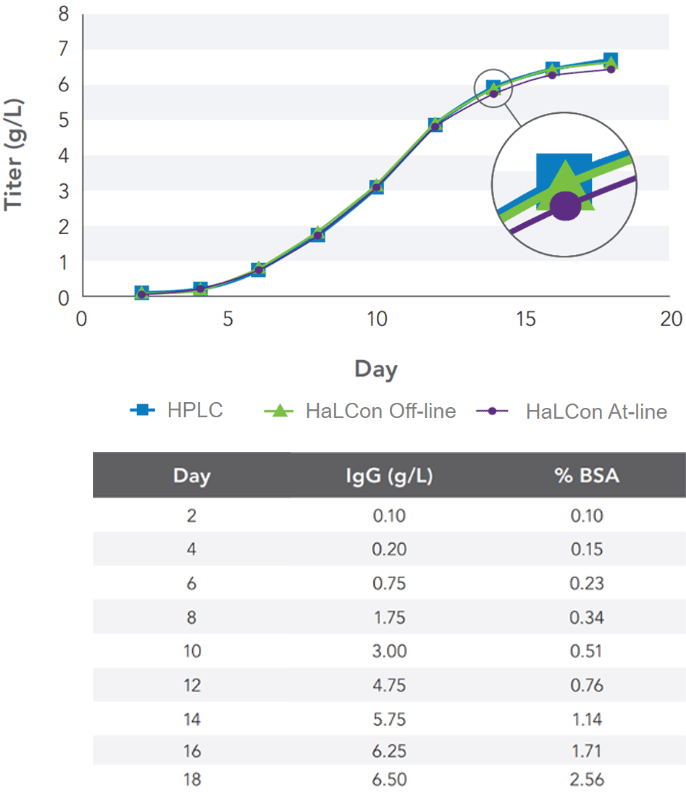
The analyzer yielded accurate at-line and off-line titer measurements compared to HPLC for titers up to 6.5 g/L without the need for sample dilution (Figure 3). Antibody titer at each sampling point was calculated using the average of three measurements for all data sets. The measurement results were also unaffected by changing BSA levels up to 2.5% (v/v).
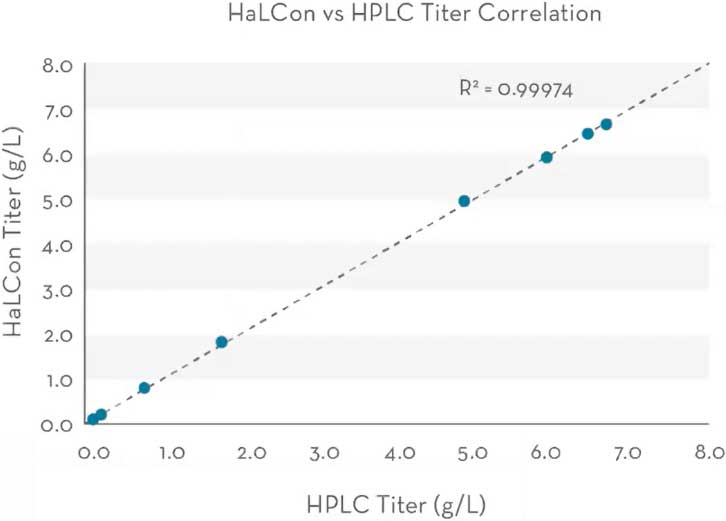
The titer measurements exhibited strong correlation with HPLC titer measurements throughout the titer range, as demonstrated by an R2 value of 0.99974 (Figure 4).
Comparability to HPLC and BLI
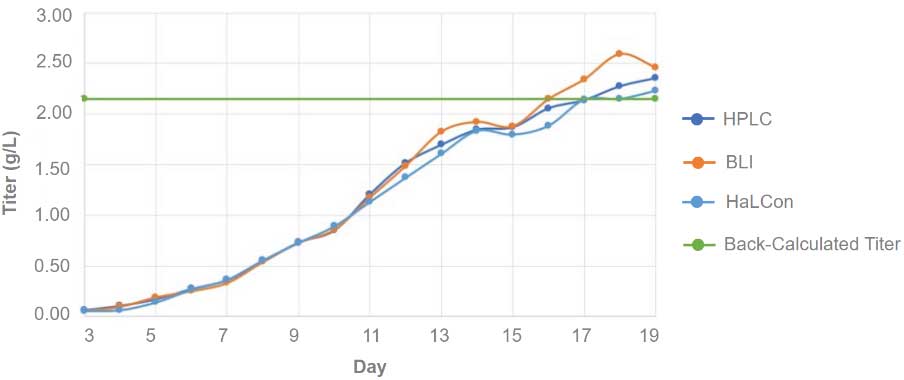
In a separate study, the performance of the HaLCon was assessed by comparing it to traditional off-line HPLC (Agilent 1100 HPLC) and BLI (ForteBio Octet RED96) methods for monitoring protein titer during a bioreactor manufacturing run using a stable CHO cell line expressing a humanized IgG antibody, Adalimumab4. The antibody titer was measured throughout the 19-day culture period, with the average of three daily measurements plotted in Figure 5. Protein titer at harvest was subsequently back-calculated following Protein A purification.
The titer measurements for Adalimumab obtained using the HaLCon and HPLC instruments exhibited a high degree of comparability throughout the entire bioreactor run, with values falling within of +/-5% of each other. Although the BLI data also displayed overall correlation, larger differences were observed
in comparison to the other two instruments. The larger discrepancies could be attributed to the need for multiple sample dilutions prior to BLI analysis.
The data derived from these studies show the capability of both traditional HPLC instruments and the HaLCon Analyzer to generate exceptional titer data. However, the differentiating factor lies in the HaLCon Analyzer’s purposeful design for application within the manufacturing suite. Optimized to provide simple, real-time, and dependable HPLC-level titer data with unparalleled efficiency, it is a suitable alternative to traditional HPLC in bioprocessing settings, providing the industry with a precise tool for titer measurement.
As the biopharmaceutical industry continues to face the growing demands for faster drug development and cost-efficiency, the importance of robust analytical methods is taking center stage. The ability to generate real-time, actionable data in minutes provides invaluable insights that enhance process control, support the effective design of experiments (DOE) as part of PAT, and facilitate the development of predictive models. Users can make critical decisions earlier to maximize bioreactor output and address operational bottlenecks to save time and money. Innovations like the HaLCon Analyzer mark the evolution of fit-for-purpose analytical tools, designed to meet the challenges of modern-day bioprocessing applications.
This article was originally published in the eBook
Monoclonal Antibody Manufacturing Trends, Challenges, and Analytical Solutions to Eliminate Bioprocessing Bottlenecks.
You can download all the articles in the series, by downloading the eBook.
Footnotes
-
1. Guidance for Industry PAT – A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance, Food and Drug Administration (FDA), 2004.
-
2. David Sloan. Introduction to the HaLCon Protein Analyzer [Video]. YouTube. https://youtu.be/UFMlRTrcmuo?si=ZLjamgsSLBLob4rp Published October 30, 2023. (accessed October 30, 2023).
-
3. RedShiftBio. HaLCon Product Brochure. May 12, 2023. https://www.redshiftbio.com/resources/halcon-brochure (accessed October 30, 2023).
-
4. Valerie I. Collins, Marisa Pisani, David J. Sloan, and Richard Huang. HaLCon, a Fit for Purpose, Platform Method for Rapid Antibody Concentration Measurements from Cell-Free Culture Media. Poser presented at Bioprocessing Summit; August 14, 2023; Boston MA. https://www.redshiftbio.com/resources/halcon-a-fit-for-purpose-platform-method-for-rapid-antibody-concentration (accessed October 30, 2023).