STEP Technology for the rapid generation of very high producing stable CHO cell lines
Introduction
Two key drivers in cost of goods for biopharmaceutical manufacturing are product yield and efficiency of manufacturing. Included in this efficiency is the time it takes to generate a commercial cell line with the kind of yield and product quality suitable for advancement to process development then manufacturing. Due to the success of CHO cells and their popularity in biopharmaceutical manufacturing, advancements in the production capabilities of these cells is fundamental to improving biomanufacturing productivity.
To further these efforts, Batavia Biosciences has created a new technology called STEP, which enables the rapid generation of high producing stable mammalian CHO cell lines.
Menzo Havenga (CEO) explains: “Yield from mammalian cells in bioreactors is critical to control cost of goods and STEP technology allows us to make at least 10-fold more protein per cell”. Timothy Smith (commercial Director) continues; “There are many diseases for which there is no treatment simply because the production costs are too high. Our STEP technology paves the way for making such medicines cost efficiently”.
STEP technology; how does it work?
The STEP technology provides a platform for production of recombinant proteins and antibodies in mammalian cells. Hereto, STEP plasmids are equipped with elements that allow very efficient and flexible cell selection under high antibiotics pressure. Inventor Femke Hoeksema (Team leader Batavia Biosciences); “STEP combines a system in which ribosomal translation of a crippled antibiotics resistance marker is attenuated hence a cell needs to make huge amounts of resistance marker mRNA in order to survive antibiotics pressure. Next, we coupled this selection system to the gene of interest (for instance Erythropoietin), ensuring that also the protein of interest is highly expressed in surviving cells. Finally, we discovered novel genetic enhancer elements that boost expression and these elements flank the cell selection system on the STEP-plasmids”. Femke continues by explaining that STEP technology also utilizes different mutated antibiotics resistance markers such that they are more- or less crippled. This allows selection of CHO cells that express the protein of interest at the highest possible level without compromising cell growth in bioreactors. Since STEP technology only yields few very high expressing cell lines, the time consumption and thus cost in manufacturing STEP cell lines is significantly reduced as compared to other stable cell line generation technologies.
STEP technology; a case-study
One of the proteins expressed with STEP technology is Erythropoietin (EPO), a recombinant protein used in the treatment of anemia, and known to be very complex as it requires extensive sugar structures in order to be biologically active. Stable STEP-CHO cell lines were obtained within a timeframe of 10 weeks and proved impressive as regards EPO yields as witnessed in both batch- and Fed-batch culture in bioreactors (see figure A + B).
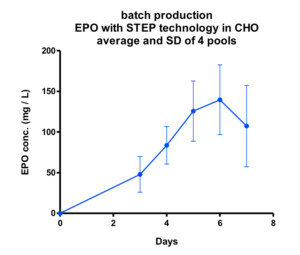
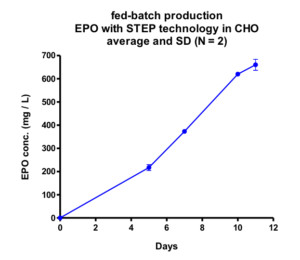
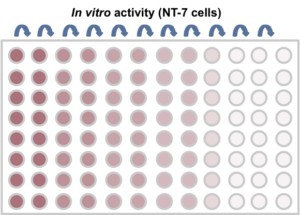
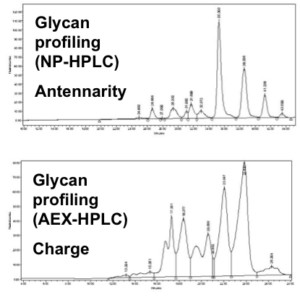
The EPO cell lines demonstrated a 600 mg per liter EPO production on day 11 in a non-optimized, generic fed batch process. The EPO protein produced with STEP technology proved biologically active (Figure C) and sialylation and glycosylation patterns (Figure D) proved identical to the EPO biological reference standard.
Menzo Havenga concludes: “such data demonstrate that we are truly pushing the boundaries of protein manufacturing and as such we see that our technology provides a huge STEP forward.