T Cell Media: A Comprehensive Guide to Key Components
Alyssa Master, PhD, Head of Operations, Nucleus Biologics
Roddy S. O’Connor, PhD , Research Assistant Professor, Center for Cellular Immunotherapies, University of Pennsylvania
With the recent surge of cellular T cell-based immunotherapies, more and more researchers are taking an interest in these specialized immune cells. Whether you are brand new to the field or an experienced immunologist, learning to work with a new cell type can often feel overwhelming. In this guide, we will explain the ins and outs of culturing T cells, so you can spend less time searching for answers and more time making discoveries.
T Cells: Key Players in Adaptive Immunity
The immune system has two main branches; nonspecific “innate immunity” and specific “adaptive immunity”. T cells, along with B cells, are responsible for adaptive immunity where cell-mediated responses and antibody responses are targeted against a specific pathogen. When T cells are exposed to their target antigen by an antigen presenting cell (APC) such as a dendritic cell or macrophage, it sets in motion a cascade of activation, proliferation, and differentiation to cytotoxic effector cells, making T cells a desirable cell type for cancer immunotherapy.
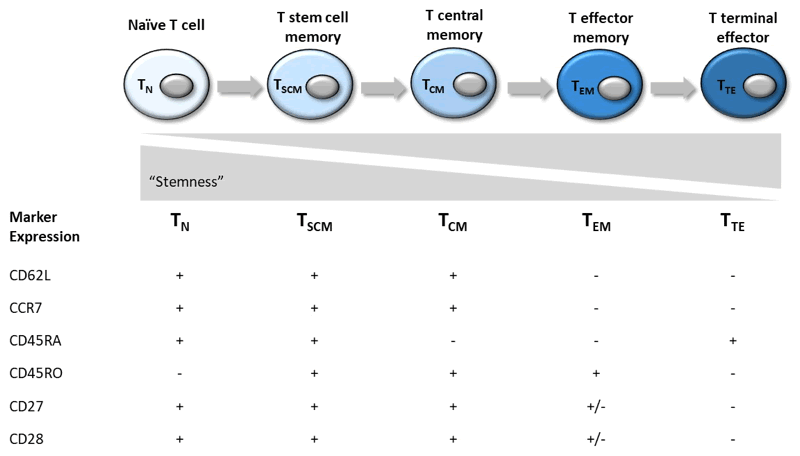
The two major arms of the T cell system include the CD4+ T helper (Th) and the CD8+ T cytotoxic (Tc) populations, which can be further broken down by their expression profiles 1. CD4+ T helper cells include the subsets Th1, Th2, Th9, Th17, Th22 and Treg (regulatory). Cytotoxic CD8+ T cells are the main effectors of cell-mediated adaptive immune responses and can be divided into the categories listed in Table 1. These subsets exhibit a spectrum of decreasing stemness and increasing effector function as they become more differentiated from naïve (TN) to effector T cell (TTE), and can be easily identified by the markers CCR7 and CD45RO.
Adoptive T Cell Therapy (CAR-T)
The concept of chimeric antigen receptor T cells (CAR-T) was first described in 1989 by Eshhar et al2, and the first FDA-approved therapy was pioneered by University of Pennsylvania years later. There has been a recent surge of commercial interest for T cell-mediated therapies due to the FDA approvals of two CAR-T therapies in 2017, Kymriah® and Yescarta™ for the treatment of acute lymphoblastic leukemia and lymphoma. In the current CAR-T manufacturing process, T cells harvested from the patient (autologous) are genetically engineered to express chimeric antigen receptors (CARs) specific to the cancer cells. These modified cells are then culture-expanded and re-infused back to the patient in a process called adoptive cell transfer (ACT).
CAR-T Cell Media Requirements
This CAR-T cell manufacturing process requires rapid activation, CAR transduction, and expansion ex vivo, underscoring the critical need for effective culture methods and consistent materials. An effective CAR-T cell manufacturing process involves media formulations that create highly potent end products. While the initial emphasis was on ex vivo proliferation capability, several researchers have found that maintaining naïve and central memory phenotypes during manufacturing is highly correlated with better clinical outcomes3. In addition, the CAR construct and subsequent transduction can be a very expensive part of manufacturing. The composition of the media can have a marked effect on transduction efficiencies which can, in turn, affect the cost and potency of the final product. Modulation of CAR-T cell phenotype and transduction efficiency is possible with novel media formulations4.
The Basics of T Cell Culture
In research laboratories, whole blood from donors is the most common starting material to isolate T cells. First, peripheral blood mononuclear cells (PBMCs) are separated from the other blood components (e.g. red blood cells and plasma). T cells are then further isolated from the other mononuclear cells using positive or negative antibody-based magnetic bead selection. For CAR-T therapies, leukocytes are isolated from the patient directly via leukapheresis and enriched for lymphocytes using counterflow centrifugation5. In both cases, once isolated, the T cells are ready for culture expansion and manipulation.
The type of culture media for successful cultivation can vary depending on the type of cell or subset of cell you require. For T cells, interleukin-2 (IL-2) is a potent cytokine which modulates proliferation and differentiation into effector and memory T cells. Culture conditions may be further refined to polarize T cells to a specific phenotype during expansion. For example, IL-4, IL-7 and IL-15 have been reported to be essential for induction, survival or turnover of memory T cells, respectively6.
For proper T cell function, activation through antigen presentation is required. However, purifying autologous APCs to use for ex vivo activation can be expensive and labor intensive making it difficult to obtain a potent CAR-T cell product. Alternative activation methods have been developed, including cell-based, bead-based, and antibody-based activation5. Magnetic beads coated with anti-CD3/anti-CD28 monoclonal antibodies to activate T cells have several advantages over cell-based aAPCs including ease of removal through magnetic separation and ability to standardize conditions.
T Cell Media
There are many choices for T cell culture media available to researchers, ranging from DIY recipes to commercially available “all-in-one” complete formulations. There is little consensus in the field as to which formulation is best making the choice of culture media a difficult one. At a minimum, T cell media includes a buffer system, protein, trace elements, vitamins, inorganic salts, and energy sources. Many formulations contain or require addition of IL-2, a cytokine important for T cell expansion. Perhaps the most commonly used media for T cell expansion is RPMI 1640 basal medium supplemented with 10% fetal bovine serum (FBS). While FBS is a robust additive supporting the culture of many different cell types, its inclusion in culture media may be undesirable for certain applications.
For T cell culture, there has been a shift in the field towards animal-free, more defined media formulations with better consistency, traceability and regulatory compliance as researchers move forward with an eye towards cell therapy. In the world of cell culture media, there are standard terms used to define the level of purity of the ingredients, though often these terms are misunderstood or misused. For instance, xeno-free media is gaining ground in the T cell field, but what does that really mean? Xeno-, is a Greek prefix meaning “foreign”, indicating that the product does not contain non-human components. Unfortunately, FBS, the gold standard media supplement, is one such offender, and many researchers are now searching for human-origin alternatives to use in their culture media. Several xeno-free serum replacements are available on the market now.
Chemically-defined media provides some advantages, namely that the composition and concentration of all the components are known. However, the tradeoff is that cells often do not grow as well in these more defined, less robust conditions. For adoptive T cell therapy, patient-derived T cells failed to grow optimally in serum-free media and exhibited reduced efficacies of gene transfer resulting from suboptimal T cell activation7, which is likely not an acceptable practice. Therefore, it is important to evaluate your needs prior to selecting a media type that is right for you.
Table 1. Cell Culture Media Types and Definition
| Media Type | Description |
|---|---|
| Serum-containing | Serum-containing media contains serum or plasma, which contains nutrients required for cell survival and growth including growth factors, hormones, macromolecules, and adhesion factors. The most commonly used serum is fetal bovine serum (FBS). |
| Serum-free | Serum-free media does not contain serum or plasma though it may contain components derived from serum or plasma. It may contain animal-derived components such as bovine serum albumin (BSA). |
| Xeno-free | Xeno-free media does not contain any animal-derived (non-human) components. Xeno-free medium may contain human-derived components, such as human serum, growth factors or insulin. |
| Chemically-defined | Chemically-defined media has components that are of a known chemical structure and concentration. |
T Cell Media Components: What are they and what do they do?
Most cell culture media lack an ingredients list and oftentimes the type of media a researcher chooses depends on the intended application. For example, the most widely used medium for culturing T cells in research laboratories is RPMI 1640 supplemented with FBS, whereas for the biomanufacturing of T cells for adoptive cell therapy, “complete” formulations such as X-VIVO 15 (Lonza, Inc) and CTS OpTimizer (Thermofisher, Inc) supplemented with human serum are more common7.
So, what exactly is in T cell media and how does it help T cells grow? We break down the most common media components below (Table 2) and explain their roles.
| Component | Function |
|---|---|
| Sodium Bicarbonate (NaHCO3) | A non-toxic natural buffer commonly included in stem cell medium to stabilize changes in pH within a CO2 incubator. |
| HEPES (4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid) | A zwitterion that acts as an organic buffer to stabilize changes in pH in a more robust manner compared to sodium bicarbonate. |
| Phenol Red | A visual pH indicator. It is yellow when media pH is below pH 6.8 and fuchsia above pH 8.2. |
| Protein | Proteins can act as carriers, offer media stability, and provide protection of cells against physical damage. The most commonly added proteins are albumin, transferrin, fibronectin and insulin. |
| Amino acids | Supplemented in cell culture media to replace those depleted during logarithmic growth phase. They are building blocks required for protein synthesis in cells. |
| Carbohydrates | Carbohydrates are the main energy source for cells in culture media. Glucose is the important for T cell proliferation. |
| Lipids | Lipids are necessary for cell membrane synthesis and activate important signaling pathways. |
| Inorganic Salts | Inorganic salts in the media help maintain osmotic balance and regulate membrane potential by providing sodium, potassium, and calcium ions. |
| Trace Elements | Trace elements such as zinc, copper, selenium and tricarboxylic acid intermediates are commonly added to culture media. They serve as enzyme cofactors. |
| Vitamins | Vitamins cannot be synthesized in sufficient quantities by cells and are necessary supplements in culture media for cell growth and proliferation. |
| ß-mercaptoethanol | ß-mercaptoethanol (ß -ME or 2-ME) is an antioxidant that reduces oxidative stress through removal of free radicals from the culture environment. Required for growing murine T cells. |
| Growth Factors | Interleukin-2 (IL-2) is an important cytokine for T-cell proliferation and is often added to T cell media. |
Table 2. Common T Cell Media Components and their Functions
Buffering System
Cells have a narrow physiologically acceptable pH range that they require their culture environment to fall within, usually between 7.0-7.4 but that can vary by cell type. T cells can be particularly sensitive to pH and it has been shown that neutral (7.0) and acidic pH can severely inhibit activation8. Buffer systems are used in media formulations to monitor and maintain pH levels.
Culturing cells in normoxic conditions (37°C, 5% CO2, 95% humidity) allows gaseous buffering of the media’s sodium bicarbonate content. This so-called “natural” buffering system approximates physiological conditions.
H2O + CO2 ↔ H2CO3 H+ + HCO3¯
NaHCO3 Na+ + HCO3¯
Another common approach is buffering with HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), an organic zwitterion that can modulate pH independent of CO2 levels (useful in hypoxic culture conditions) 9. It can be used in combination with sodium bicarbonate with pH sensitive cell types, such as T cells, that require increased buffering capacity.
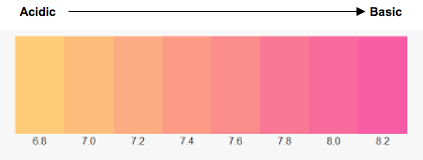
A common method to monitor pH in cell culture media is through the inclusion of phenol red. Its color changes depending on the pH, where media below pH 6.8 (acidic) appearing yellow and above pH 8.2 (basic) appearing fuchsia (Figure 2). Activated T cells shift their metabolism to aerobic glycolysis which culminates in lactic acid production. At physiologic pH lactic acid dissociates into its corresponding [H+] and lactate anion which are exported. This results in extracellar acidification in metabolically active T cells. Phenol red provides an easy way to see if the pH of your culture is within a healthy range or needs to be changed.
Protein
Proteins in culture media have a multitude of functions from acting as carriers to protecting cells against physical damage and offering media stability.
Serum
In the manufacture of T cell therapies, human serum (HS) is a common additive to T cell media (i.e. X-VIVO 15 + 5% HS). HS is preferred over FBS, since FBS is of animal origin. However, HS is expensive and there is variability in quality, availability and efficacy. This demands/requires frequent screening to compare the impact of different lots on T cell survival and expansion.
The current practice for adoptive T cell therapies is to supplement media with 5% human serum, which has been feasible to do for proof-of-principle studies up to phase III trials. However, it is very likely that the HS supply will become scarce as demand increases and will soon be a rate limiting reagent as more therapies near US Food and Drug Administration (FDA) approval7. Therefore, a T cell manufacturing process independent of human serum that is cGMP compliant would be an important step to make adoptive T cell therapy less expensive, more consistent, and available to more patients10.
Alternatives for FBS and HS include serum replacements such as PhysiologixTM XF SR (Nucleus Biologics) that utilize cGMP grade, xeno-free growth factor and cytokine mixtures to deliver optimal performance while maintaining favorable T cell phenotypes and enhancing transduction efficiency.
Insulin
The hormone insulin and the downstream signaling through its receptor (INSR) has been shown to have a great impact on adaptive immune function by modulating T cell metabolism. T cell activation is an energy-demanding process fueled by increased glucose consumption. This process is accompanied by upregulation of INSR, which supports T cell nutrient uptake and associated glycolytic and respiratory capacities allowing them to acquire full effector functions11,12.
Transferrin
Transferrin plays an essential role in normal early T-cell differentiation in vivo, a role attributed to its iron transport function13. Moreover, the transferrin receptor (TfR) was found to be upregulated during T cell activation after the interaction of the T cell receptor with the antigen-major histocompatibility complex and the expression of IL-2 receptor14.
Amino acids
Amino acids are the building blocks of proteins and facilitate the storage and transfer of nitrogen to the cells in culture. Cells can produce non-essential amino acids (NEAA), but may not produce enough to replenish those depleted during rapid growth. Adding supplements of NEAA to media can both stimulate growth and prolong the viability of the cells in culture. ‘Essential’ amino acids, however, cannot be synthesized so they must be added to culture media for cells to proliferate.
L-glutamine is an essential amino acid, and is a major fuel for many cells including lymphocytes and macrophages ex vivo. The concentration of extracellular glutamine appears to regulate T cell proliferation, IL-2 production and IL-2 receptor expression with the ideal concentration range being 0.6-2.0mM for lymphocytes15.
An important consideration when using glutamine for culture media is its instability in aqueous solution. It rapidly degrades at 37°C resulting in undesirable ammonia buildup, which can be detrimental to cells. This is why many commercially available media are formulated without L-glutamine, requiring its addition at the time of use. To circumvent this, media can be supplemented with L-glutamine in dipeptide forms, such as alanyl-l-glutamine and glycyl-l-glutamine, which are more stable and less prone to degradation.
Carbohydrates
The main source of energy for cells is derived from carbohydrates in the form of sugars. Glucose and galactose are the most common additives; however, some media contain maltose or fructose9.
Resting T cells can meet 96% of their energy demand through oxidative phosphorylation but will switch to aerobic glycolysis upon activation; this is known as the Warburg effect11. Following T cell activation by APCs, CD28 signaling leads to an upregulation of glucose transporter (Glut)1 and an increased activity of several glycolytic enzymes16,17. Interestingly, glucose is not equally important for all T cell subsets. Effector T cells strongly increase glycolysis after activation while regulatory T cells operate in a glucose-independent manner because they use fatty acid oxidation for energy production17.
Lipids
Fatty acids serve as fuel for cells but are also precursors to produce cholesterol and membrane phospholipids. Studies have shown that T cell development, differentiation, migration, function, and survival are influenced by lipid metabolism where low concentrations of fatty acids can increase the proliferation of T cells while higher concentrations induce apoptosis in a dose-dependent manner18,19.
Inorganic Salts
Inorganic salts, such as calcium, magnesium and potassium are important for regulating the osmotic balance9. They also release ions which regulate membrane potential and serve as cofactors for enzymes. Calcium functions in T cell activation and also modulates the unique metabolic changes that occur in distinct T cell subsets and developmental stages20.
Trace elements
Trace elements such as zinc, copper, selenium and tricarboxylic acid intermediates are commonly added to culture media9. Through its incorporation into selenoproteins, selenium (Se) is involved primarily in regulating oxidative stress and redox in nearly all tissues and cell types, including those involved in innate and adaptive immune responses21,22,23.
Selenium exerts differential influences on T cell maturation and activation based on the type of antigens to which they are exposed and on their susceptibility to redox status. For example, Se supplementation may enhance Th1-type immune responses to a greater extent than Th2-type responses21.
Vitamins
Vitamins are precursors for numerous co-factors and many are necessary for cell growth and proliferation, especially B group vitamins. The vitamins commonly added in media are riboflavin, thiamine and biotin9.
The switch from naïve T cells to effector T cells requires the presence of sufficient extracellular vitamin D24. When T cells are exposed to a foreign antigen, upregulation of the vitamin D receptor occurs. Without sufficient extracellular vitamin D present, the T cells are unable to make the transition to effector T cells.
Vitamin A is also known to play a role in the immune system, through its metabolite retinoic acid (RA). RA induces expression of gut homing receptors on T and B cells, allowing the migration of these cells to the intestine, and also to the inflamed tissues, to perform their effector functions22. In addition, vitamin A has been shown to be essential for T cell activation and differentiation into T helper subsets Th1, Th2 and Th17 cells26.
ß-Mercaptoethanol (2-Mercaptoethanol, 2-ME, ß-ME)
Also referred to as BME, 2-ME this chemical acts as a reducing agent to maintain the intracellular redox environment. Particularly for T cells grown in serum-free conditions, the addition of 2-ME was found to promote T cell proliferation in vitro27.
Interleukins
Interleukin-2 (IL-2) is an important cytokine for T cell culture. T cells produce IL-2 following antigen presentation, which then stimulates their growth, differentiation, and survival. It is such a potent stimulator of cytotoxic T cells that IL-2 alone was approved as a therapy for two types of cancer. Concentrations of IL-2 in media vary wildly in the literature; from 10 to 600 IU/mL. While higher doses result in improved proliferation, studies have found that it can also reduce cytotoxicity. Fine tuning of the IL-2 concentration during ex vivo expansion of T cells can yield high numbers of T cells with the desired characteristics for adoptive immunotherapy28. Optimization experiments should be conducted to determine which concentration works best for your particular system.
IL-7 and IL-15 are also important for T cell growth and function. IL-7 and IL-15 are enriched in the lymph node and support the survival of memory T cells. Thus, IL-7 and IL-15 are frequently used at around 10 ng/mL each, instead of IL-2, for generating central memory T cells or less differentiated progeny29.
Antibiotics
Antibiotics can be added to culture media to prevent contamination of cells. However, many labs choose not to use them because they can mask low levels of contamination and have unwanted/unexpected effects on your cells. The presence of antibiotics can interfere with cell metabolism and alter cell gene expression profiles, which can confound experimental results30. They are certainly not necessary for cell culture if proper aseptic technique is utilized so you can decide whether or not to include antibiotics in your media.
Final Remarks
It is important to understand the components of your T cell media because there are many factors that can influence the activation and differentiation of these cells, which can in turn determine the success of your T cell culture. Because their metabolism is so closely linked to their survival, differentiation, and activation, there is tremendous interest in understanding and manipulating metabolic processes for therapeutic intent31.
Adoptive cellular immunotherapies are an exciting and paradigm-shifting modality for cancer treatment and undoubtedly interest in T cells will continue to rise. We hope this guide will serve as a useful tool for anyone interested in exploring T cell culture.
About Nucleus Biologics
Nucleus Biologics is leading the movement to precision cell culture with products for scientists in academia, biotechnology, and pharmaceutical companies. The company supports scientific reproducibility by commercializing innovative products using a purposeful and documented supply chain.
Footnotes
-
1. Golubovskaya V, Wu L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers (Basel). 2016;8(3):36. doi:10.3390/cancers8030036
-
2. Gross G, Waks T., and Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989; 86:10024–28.
-
3. Fraietta JA, Lacey SF, Orlando EJ, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018; 24:563-571. doi: 10.1038/s41591-018-0010-1
-
4. O’Connor RS, Ghassemi S, Schneider D, Karimi-Naser L and Master AM. A Novel Media Supplementation Strategy for Improved T Cell Culture and Preservation of Naivety. Paper presented at: International Cancer Immunotherapy Conference; October 2018; New York, NY.
-
5. Levine BL, Miskin J, Wonnacott K, Keir C. Global Manufacturing of CAR T Cell Therapy. Mol Ther Methods Clin Dev. 2016; 4:92-101. doi: 10.1016/j.omtm.2016.12.006
-
6. Lee JB, Lee KA, and Chang J. Phenotypic changes induced by IL-12 priming regulate effector and memory CD8 T cell differentiation. Int Immunol. 2007: 19(9):1039-48. doi: 10.1093/intimm/dxm072
-
7. Medvec AR, Ecker C, Kong H, Winters EA, Glover J, Varela-Rohena A, Riley JL. Improved expansion and in vivo function of patient T cells by a serum-free medium. Mol Ther Methods Clin Dev. 2017; 8:65-74. doi: 10.1016/j.omtm.2017.11.001.
-
8. Bosticardo M, Ariotti S, Losana G, Bernabei P, Forni G, Novelli F. Biased activation of human T lymphocytes due to low extracellular pH is antagonized by B7/CD28 costimulation. Eur J Immunol. 2001; 31:2829-38.
-
9. Arora M. Cell culture media: a review. MATER METHODS 2013; 3:175. doi: 13070/mm.en.3.175
-
10. Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016; 3:16015. doi: 10.1038/mto.2016.15.
-
11. Fischer HJ, Sie C, Schumann E, Witte AK, Dressel R, van den Brandt J and Reichardt HM. The insulin receptor plays a critical role in T cell function and adaptive immunity. J Immunol. 2017;198 (5): 1910-20. doi: 10.4049/jimmunol.1601011
-
12. Tsai S, Clemente-Casares X, Zhou AC, Lei H, Ahn JJ, Chan YT, Choi O, Luck H, Woo M, Dunn SE, Engleman EG, Watts TH, Winer S, Winer DA. Insulin receptor-mediated stimulation boosts T cell immunity during inflammation and infection. Cell Metab. 2018 doi: 10.1016/j.cmet.2018.08.003
-
13. Macedo MF, de Sousa M, Ned RM, Mascarenhas C, Andrews NC, Correia-Neves M. Transferrin is required for early T-cell differentiation. Immunology. 2004; 112(4):543-9.
-
14. Bayer AL, Baliga P, Woodward JE. Transferrin receptor in T cell activation and transplantation. J Leukoc Biol. 1998; 64(1):19-24.
-
15. de Oliveira DC, da Silva Lima F, Sartori T, Antunes Santos AC, Rogero MM and Fock RA. Glutamine metabolism and its effector on immune response: molecular mechanism and gene expression. Nutrire. 2016; 41:14. doi 10.1186/s41110-016-0016-8
-
16. O'Connor RS, Guo L, Ghassemi S, et al. The CPT1a inhibitor, etomoxir induces severe oxidative stress at commonly used concentrations. Sci Rep. 2018;8(1):6289. doi:10.1038/s41598-018-24676-6
-
17. Palmer CS, Ostrowski M, Balderson B, Christian N, Crowe SM. Glucose metabolism regulates T cell activation, differentiation, and functions. Front Immunol. 2015; 6:1. doi:10.3389/fimmu.2015.00001
-
18. Howie D, Ten Bokum A, Necula AS, Cobbold SP, Waldmann H. The Role of Lipid Metabolism in T Lymphocyte Differentiation and Survival. Front Immunol. 2018; 8:1949. doi:10.3389/fimmu.2017.01949
-
19. de Jong AJ, Kloppenburg M, Toes RE, Ioan-Facsinay A. Fatty acids, lipid mediators, and T-cell function. Front Immunol. 2014; 5:483. doi:10.3389/fimmu.2014.00483
-
20. Toldi, G. The regulation of calcium homeostasis in T lymphocytes. Front Immunol. 2013; 4:432. doi:10.3389/fimmu.2013.00432
-
21. Hoffmann PR, Berry MJ. The influence of selenium on immune responses. Mol Nutr Food Res. 2008;52(11):1273-80.
-
22. Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16(7):705-43.
-
23. Carlson BA, Yoo MH, Shrimali RK, et al. Role of selenium-containing proteins in T-cell and macrophage function. Proc Nutr Soc. 2010;69(3):300-10.
-
24. Konijeti GG, Arora P, Boylan MR, et al. Vitamin D Supplementation Modulates T Cell-Mediated Immunity in Humans: Results from a Randomized Control Trial. J Clin Endocrinol Metab. 2015;101(2):533-8.
-
25. Bono MR, Tejon G, Flores-Santibañez F, Fernandez D, Rosemblatt M, Sauma D. Retinoic Acid as a modulator of T cell immunity. Nutrients. 2016;8(6):349. doi:10.3390/nu8060349
-
26. Ross CA. Vitamin A and retinoic acid in T cell-related immunity. Am J Clin Nutr. 2012;96(5):1166S-72S.
-
27. Click, RE. Review: 2-mercaptoethanol alteration of in vitro immune functions of species other than murine. J Immunol Methods. 2013;402(1-2):1-8.
-
28. Besser MJ1, Schallmach E, Oved K, Treves AJ, Markel G, Reiter Y, Schachter J. Modifying interleukin-2 concentrations during culture improves function of T cells for adoptive immunotherapy. Cytotherapy. 2009;11(2):206-17. doi: 10.1080/14653240802590391
-
29. Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, Bondanza A, Bordignon C, Peccatori, J., Ciceri, F., Lupo-Stanghellini, M. T., Mavilio, F., Mondino, A., Bicciato, S., Recchia, A., & Bonini C. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121(4): 573-584. doi: 10.1182/blood-2012-05-431718.
-
30. Ryu AH, Eckalbar WL, Kreimer A, Yosef N, Ahituv N. Use antibiotics in cell culture with caution: genome-wide identification of antibiotic-induced changes in gene expression and regulation. Sci Rep. 2017;7(1):7533. doi:10.1038/s41598-017-07757-w
-
31. Iyer RK, Bowles PA, Kim H, Dulgar-Tulloch A. Industrializing autologous adoptive immunotherapies: manufacturing advances and challenges. Front Med (Lausanne). 2018; 5:150. doi:10.3389/fmed.2018.00150