
The scale-X™ carbo bioreactor: flexible GMP viral production at bench scale in adherent or suspension processes
Producing viral products for vaccines or cell and gene therapy starts with cell culture, where bioprocessing equipment must be selected to ensure conditions established at bench-scale can be successfully translated to the clinics and onwards. A successful development and manufacturing platform must result in low cost of goods for the final product, high design robustness (low risk of failure) and ease of use and scale-up.
The scale-X carbo system from Univercells Technologies enables seamless transition from development to clinical and commercial production in one piece of bioprocessing equipment. The scale-X carbo provides a flexible, highly productive, mid-scale platform to produce viral products with both adherent and suspension cell lines.
How it works
The scale-X carbo is a mid-scale fixed-bed bioreactor system that features a drastically reduced equipment footprint with an intensified cell culture process. Cells are entrapped within a structured matrix (fixed bed) while media is circulated through the bed to ensure adequate nutrient and gas supply. When the target cell density is reached, infection or transfection is carried out in-situ. An in-line concentrator controlled by the same automation system as the bioreactor enables an automatic concentration of the viral product.
The flexible 10 or 30 m² scale-X carbo bioreactor configuration places it ideally at the mid-scale between a bench-top and a large manufacturing-scale system. As such it can be used for process development as well as clinical and small-scale GMP production.
Quick hit benefits
1. Low footprint: The scale-X carbo platform is 0.36 m² tall with a working volume ranging from 1.6 to 3.2 L equality a throughput equivalent to a stirred tank bioreactor of at least 40 L. This results in a compact system which can be integrated within a laminar flow if required or operated on a bench-top, with minimal space occupation. Laboratories and cleanrooms are de-cluttered, and handling is simplified, which leads to low operational costs and reduced risks of batch failure.
2. Viral production and TFF concentration-in-one: The bioreactor is chained with an in-line hollow-fibre TFF concentrator. Both are controlled through a central automation system for continuous concentration of harvested product. The resulting low volumes help to further simplify downstream purification steps.
3. Intuitive software interface: Proprietary Wonderware based scale-X carbo software is designed to enable intuitive handling. A simple and clear process flow diagram representing the process step gives easy access to key operating parameters. Pre-defined process sequences can be configured to automate process steps such as batch or recirculation mode cell culture. The software is designed to help end-user with 21 CFR part 11 compliance with user access control, audit trail and data recording.
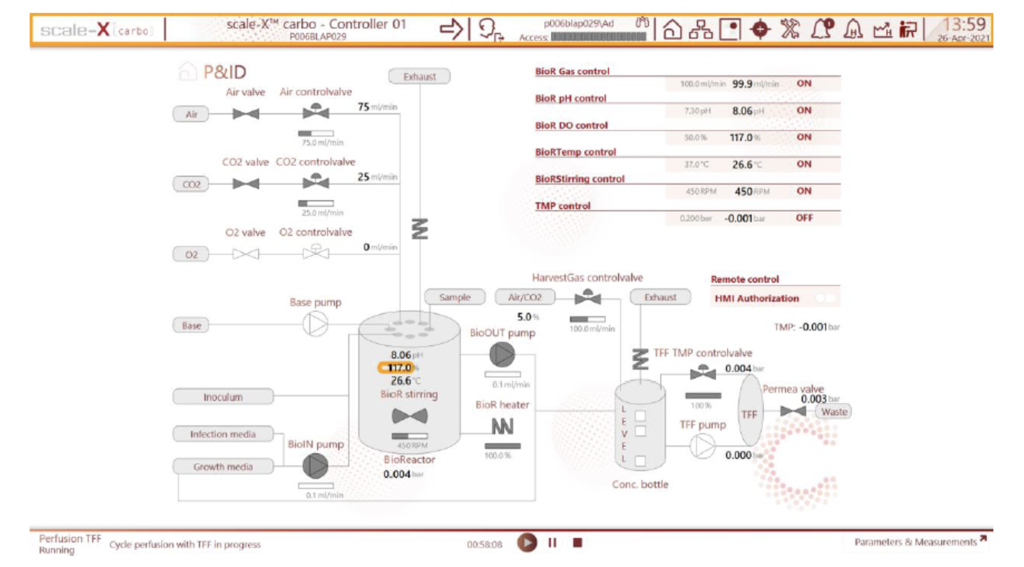
4. Higher productivity than other fixed-bed bioreactors via two unique features that result in best-in-class viral productivity:
a. Structured fixed bed → The matrix supporting cells inside the bioreactor is homogeneous both in the vertical and horizontal direction. Cells distribute and grow homogeneously through the bed which results in both high productivity and reproducible batch-to-batch performance.
b. Impeller-driven agitation → Agitation and media supply to the cells are achieved using a magnetically driven impeller located inside the bioreactor. Thanks to this, availability of nutrients and other additives is always homogeneous and independent of media supply strategy leading to consistently high and reproducible productivity.
Demonstrated superior productivity for virus & viral vector production
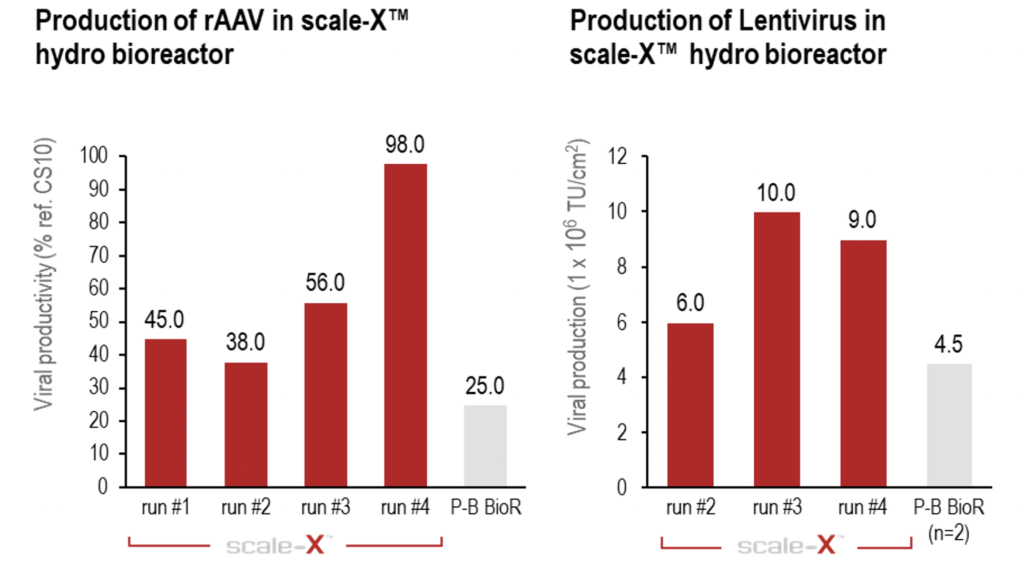
Users of the scale-X bioreactor can expect up to 4-fold higher productivity for AAV and Lentivirus (LV) in HEK293 cells compared to other fixed-bed bioreactors¹.
In the example presented, there was 2-log higher productivity for an rVSV in Vero cells than an alternative fixed-bed bioreactor recorded².
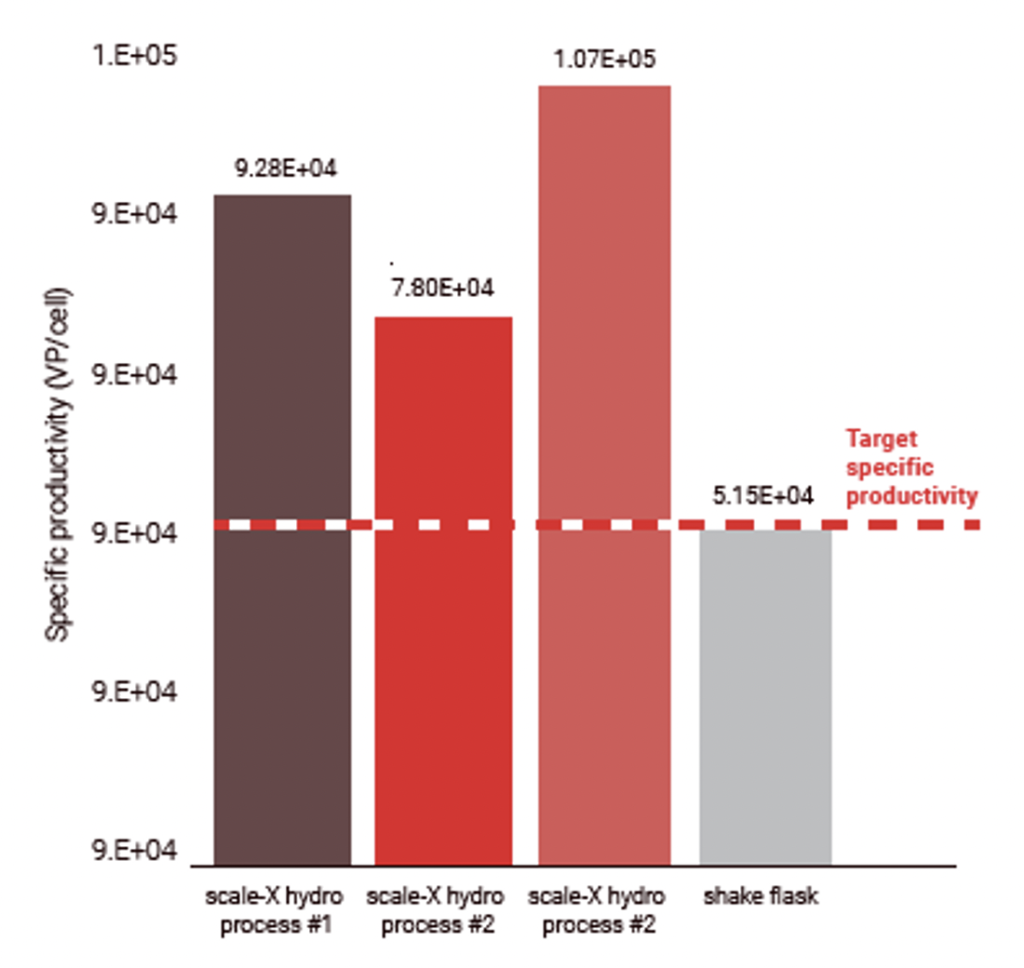
2-fold higher productivity for an Adeno virus COVID-19 vaccine in HEK293 cells was also shown when compared to an existing shake flask process².
Additionally, higher productivity was demonstrated for un-disclosed paediatric vaccines in WI-38, Vero and CEF cells, a Rubella vaccine in MRC-5 cells, an Ebola vaccine candidate3-5.
Flexible solution
The scale-X bioreactor is compatible with suspension or adherent cells in a low footprint achieved via process integration. Suspension cells can be grown within the structured fixed bed matrix and produce viral material in high quantities. A recent study demonstrates how suspension adapted HEK293 cells were successfully grown to produce a COVID-19 adenovirus vector vaccine in scale-X6.
Users can leverage the scale-X bioreactor across R&D, clinical and commercial phases with the convenience of single-use assemblies. Tubing assemblies are also available for cell culture and concentration liquid handling in both R&D and GMP versions.
To learn more about the technology, please see the scale-X carbo product page.
Or to read more about how Batavia Biosciences used the scale-X carbo to produce a Phase 1 Lassa fever vaccine candidate, visit:
https://www.bataviabiosciences.com/news/hip-vax-lassa-fever-vaccine-scale-x-bioreactor/)