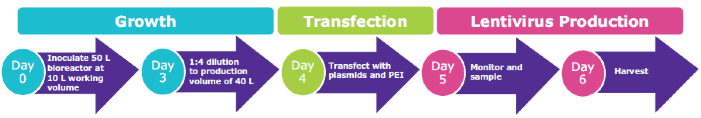
Viral Vector Process Intensification: Stepwise Model for Efficient Scale-up
The unprecedented clinical success of novel cell and gene therapies (CGT), such as CAR-T cell therapy, has led researchers to shift from treating more rare conditions present in smaller populations to those more prevalent in the general population. This has, in turn, resulted in a growing market demand for the viral vectors used in these therapies. It has highlighted the need to increase manufacturing capacity to meet market demands by developing scalable and cost-effective viral vector manufacturing processes and improving methods for purification and analysis to ensure product purity, efficacy, and safety. Research and Markets recently reported the global viral vector manufacturing market was valued at US$459.4 million in 2019 and – expected to reach US$2.2 billion by 2027.
Two of the most commonly used viral vectors for CGT are recombinant adeno-associated viruses (rAAV) and lentiviruses (LV). Currently, most lentiviral vector clinical and commercial manufacturing consists of transient transfection of four plasmids in adherent flat stock cultures using HEK293T (or derivative) cells grown in serum-containing media. While planar culture surfaces can achieve high titers and efficiencies, there are several challenges associated with using an adherent platform for large-scale lentivirus production (Figure 1). These adherent platforms are very “high-touch”, manual processes, where feeding, passaging and harvesting cells are labor intensive and costly. These factors, combined with the cost and lot-to-lot serum variability, are significant hurdles to overcome. Scale-out (increase in number of identical units) of this model will strain infrastructure capacity and will likely be insufficient to meet viral particle titer demands. These challenges faced by lentivirus manufacturers are alleviated with the development of scalable lentivirus manufacturing platforms. Such platforms need to include suspension-adapted cells grown in chemically defined medium, bioreactor process development, and scale-up (increase vessel size).
In a recent white paper published by MilliporeSigma entitled “Considerations for Bioreactor Process Development and Scale-Up for Transient Transfection-Based Lentivirus Production in Suspension”, the authors detailed the process development to scale up of the VirusExpress™ 293T Lentiviral Production Cells in a strategic, stepwise manner. The authors outlined essential considerations in the process development steps to optimize this suspension-adapted production cell line cultured in a chemically defined (CD), animal component-free medium for efficient scale up of lentivirus production.
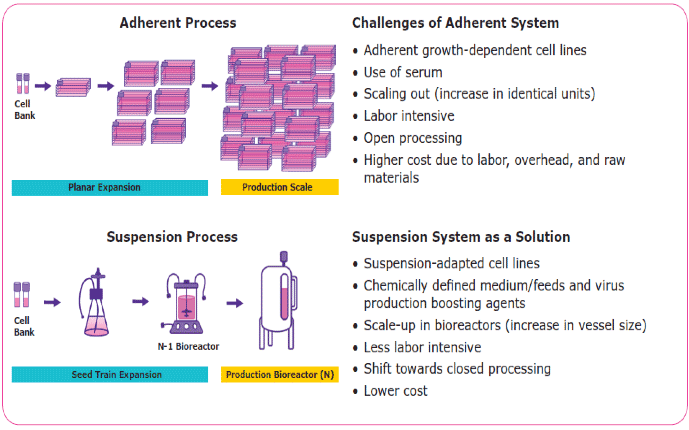
Scale Down Model
Many unit operations are involved in upstream viral vector manufacturing, including cell expansion, transfection, or infection of the vector producing cells and viral vector production. Within these unit operations, many variables such as the cell line, cell culture medium formulation, culture platform, and bioprocess parameters (temperature, pH, dissolved oxygen concentration, agitation rate, and gas sparger options) can affect cellular productivity and viral vector stability.
The investigators divided the process development into two phases, wherein Phase I, they focused on optimizing the suspension growth of the VirusExpress™ 293T lentiviral production cell line in CD medium. Once completed, Phase II focused on optimizing lentivirus production by the VirusExpress™ 293T cells. The rationale for the division was the possibility of cell growth or health being adversely affected by suboptimal growth parameters, leading to insufficient lentivirus production.
The team utilized bench-scale bioreactors, a smaller, scaled-down, cost-effective way to test changes in culture parameters before moving to larger scales, as they are predictive of cell culture behavior in larger formats. In this study, MilliporeSigma’s Mobius® 3 L Single-Use Bioreactor was used as a scale-down model for early process development activities. The vessel is a rigid, stirred tank bioreactor and contains multiple operation options, including pre-fitted weldable tubing, open pipe and microsparger options, sampling port, addition lines, a gas inlet filter, a vent filter, and a harvest line. After all the parameters were defined, scale up to a 50L bioreactor followed the same Phase I and II testing as for the scale down bioreactor.
Important Points:
- Bench-scale bioreactors should be of similar geometry to the larger scale bioreactors used so that process engineering parameters like top speed and power per unit volume can be kept constant
- It is common in bioreactor scale-up to maintain the same power per unit volume process value (W/m3) and linearly scale the process gases (maintaining the same volume of gas per volume of liquid per minute)
- Factors such as pH, agitation rate, dissolved oxygen concentration, and gas sparger options in bioreactors should be investigated and optimized based on your production cell line and culture media of choice.
Bioreactor Process Development Phase I
Process Parameter Definition for Growth of VirusExpress™ 293T Cells in CD Medium in the Mobius® 3 L Single-Use Bioreactor
In a bioreactor system, variables such as pH, agitation rate, dissolved oxygen concentration, and gas sparger options can impact cellular productivity and viral vector stability. As an example, the pH can adversely affect HEK293T cell growth and lentivirus production/stability. Therefore, these three test parameters determined the optimal conditions for the cell growth of the VirusExpress™ 293T cells. The viable cell density (VCD), nutrient consumption (i.e., glucose, glutamine), and metabolite output (i.e., ammonium, lactate) were all tested against a shaker flask controls to determine the most suitable. It is important to note that the authors included conditions in the extreme range for a particular parameter to test the system’s limits. For instance, a low pH of 6.75 in the pH excursion experiments determines cell tolerance, which helps inform their understanding of the design space and process tolerance.
Their results identified these setpoints to be optimal for VirusExpress™ 293T bioreactor cell growth:
- Agitation rate of 204 rpm (20 W/m3)
- pH of 7.05 was optimal setpoint
- An open-pipe oxygen sparger configuration was selected for gassing and to control dissolved oxygen at an optimal 50% setpoint
Bioreactor Process Development Phase II
Process Parameter Definition for Lentivirus Production by VirusExpress™ 293T Cells in CD Medium in the Mobius® 3 L Single-Use Bioreactor
After optimizing bioreactor parameters for cell growth, the authors applied the same process development principles to look at lentiviral production levels in the scale-down model.
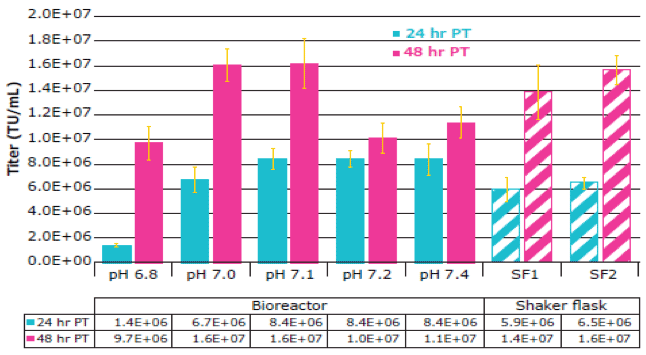
Lentivirus production was accomplished through PEI-mediated transfection using a third-generation plasmid system, expressing a GFP transgene. 48 hours post-transfection, the bioreactors were sampled for cell counts, nutrient consumption and metabolite formation, and functional titer analysis to determine the best agitation rate, pH level, and oxygen sparger setup. Their findings from the pH excursion experiments differed from published reports of optimal setpoints based on serum-containing media. The cells yielded the highest functional titer at pH 7.0 and 7.1, with a reduction of about 30–40% in titer with a slightly acidic setpoint of pH 6.8 and slightly basic setpoints of pH 7.2 and 7.4. This emphasizes the importance of performing transfection-specific optimization experiments, particularly when working with chemically defined media (Figure 2). In all cases, the setpoints identified for optimal cell growth held true for lentivirus production.
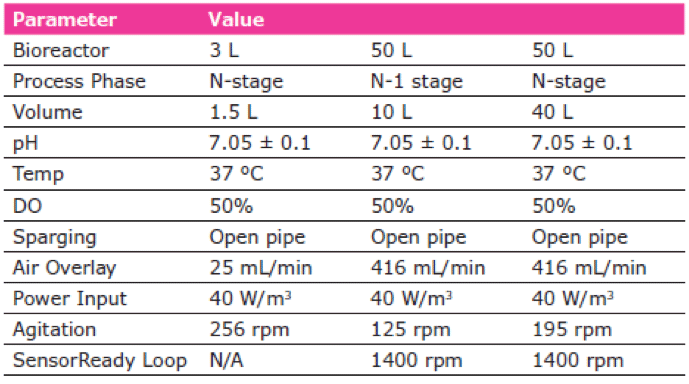
The process parameters for both the growth phase of VirusExpress™ 293T cells and lentivirus production phase defined using the Mobius® 3 L Single-Use Bioreactor (Table 1) were transferred for scale-up of activities in the Mobius® 50 L Bioreactor.
Scale-Up Phase I
Growth Characterization in CD Medium in the Mobius® 50 L Bioreactor
The scale-up studies at 10–40 L volume were conducted in the Mobius 50 L Single-Use Bioreactor. In contrast to experiments conducted in the 3L Mobius® bioreactor, both the growth (N-1 stage) and virus production (N stage) phase occurred in the same vessel (Figure 3). On day 3, once a VCD of > 4.5 x 106 cells/mL was achieved, a 1:4 dilution of the culture was performed using prewarmed, fresh media to bring the working volume to 40L for the N-stage lentivirus production.

The VirusExpress™ 293T cell growth and metabolite profiles were analyzed in a growth-only run (no cell transfection on D4) in the Mobius® 50 L Bioreactor. Concurrently, cells were grown in 250 mL shake flasks, and the Mobius® 3 L Bioreactor was used as controls. The cells achieved a VCD of 5.5 x106 cells/mL with 98.9% viability on D3.
One interesting finding is the growth profile for both N-1 and N-stages in 50 L bioreactors was slower than that of the controls. Previous data from shake flask studies indicate that a VCD of ≥ 2.4 x 106 cells/mL at the time of transfection was critical to achieving the target functional titer of > 1 x 107 TU/ mL. Because the target transfection VCD was achieved with the process parameters utilized during the growth-only run, the team decided to proceed with Phase II of the scale-up development and address the growth rate later.
Scale-up Phase II
Lentivirus Production in VirusExpress™ 293T cells and CD medium in the Mobius® 50 L Bioreactor
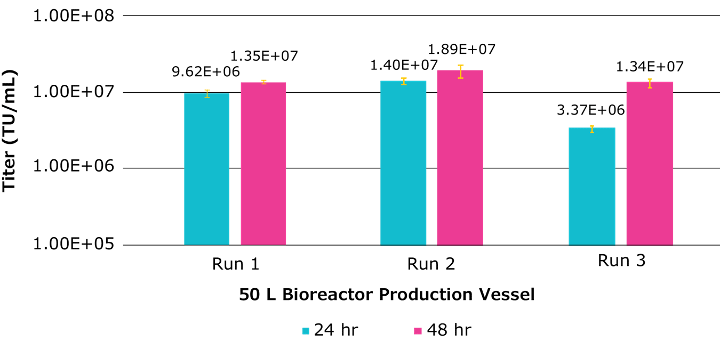
Three production runs using the Mobius® 50L bioreactor were completed to look at the process robustness and reproducibility. Figure 4 summarizes the functional titers obtained at 24 and 48hr post-transfection for each run. The results were comparable across all three production runs in the Mobius® 50 L bioreactor, all successful in exceeding a target functional titer of 1 x 107 TU/mL.
Additionally, across all three runs, a cell-specific productivity between 5 and 8 TU/cell was observed.
Concluding Remarks
Because of the limitations of adherent production cell lines, commercial manufacturing of lentivirus requires cells adapted to serum-free suspension systems that are much more amenable to scale-up. The stepwise process intensification development with the VirusExpress™ 293T Lentiviral Production Platform in the Mobius® bioreactors outlined here demonstrates an effective method for lentiviral production scale-up . Because the same bioreactor geometry exists across the Mobius® units (3L vs. 50L), process parameters can be quickly transitioned and scaled up, saving time and money. Overall, the VirusExpress™ 293T Lentiviral Production Platform consistently achieved high functional titers at a large scale, which addresses the industry’s current challenges for lentiviral production process intensification. Producing large quantities of viral vectors will help overcome the bottleneck in cell and gene therapies manufacturing.
Visit www.emdmillipore.com/VirusExpress to learn more about the VirusExpress™ Lentiviral Production Platform, to request more information, or to read the full white paper.