
Xeno-free hematopoietic cell culture medium designed for use in cell-based therapy production
Hematopoietic stem and progenitor cells (HSPCs) hold tremendous therapeutic potential for use in cell therapies to treat blood cancers and other blood diseases including sickle cell anemia and immune related disorders. HSPCs most frequently are sourced from donor blood or bone marrow. These cells are rare with only 1 in 100,000 found in peripheral blood cells and 1 in 10,000 found in bone marrow. In order to achieve the dosage required for most cell therapies (100’s of millions), cells must be successfully expanded to high numbers in culture. However during expansion, cells must also maintain the progenitor cell properties including the potential to differentiate and mature into the various hematopoietic lineage cells required for therapeutics.
It must also be considered that for Cell Therapy applications, the cells must be expanded using methods compatible with Cell Therapy manufacturing guidelines.
With this in mind, Irvine Scientific recently launched their PRIME-XV® Hematopoietic Cell Basal XSFM, a xeno-free, serum-free basal medium for human hematopoietic progenitor cell culture. The medium has been optimized to support high-level expansion while maintaining functionality and balanced lineage differentiation potential. The medium is manufactured using stringent raw material qualification and under Current Good Manufacturing Practices (cGMP) for consistency and reliability. It is designed to ease the transition from research cell culture to clinical applications.
Highlights of the new medium include:
Supports high-level expansion
Irvine Scientific presents data in Figure 1 to demonstrate the high-level expansion of hematopoietic progenitor cells derived from human cord blood. This high-level expansion is critical in reaching therapeutic dose levels. However, to be utilized in therapies, they must maintain their multipotency in culture.
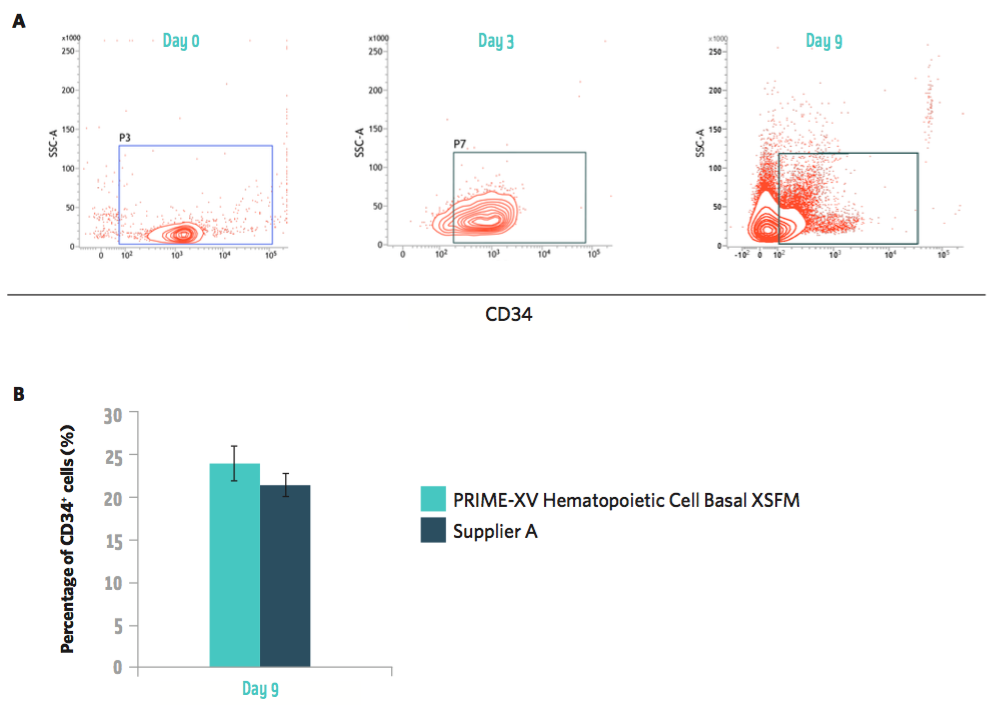
Supports maintenance of CD34+ hematopoietic progenitor cell population
One of the biggest challenges in culturing hematopoietic progenitor cells is preventing differentiation. HSPCs tend to spontaneously differentiate over time in culture, which makes it difficult to achieve high levels of multipotent cells. To address this, PRIME-XV® Hematopoietic Cell Basal XSFM has been formulated to maintain cells in their progenitor state. This is measured by expression of CD34 and differentiation potential. The ability to efficiently expand CD34+ HSPCs is critical to facilitate the progression of both allogeneic and autologous cell-based therapies toward clinical applications. In figure 2, Irvine Scientific presents data to show that by using PRIME-XV® Hematopoietic Cell Basal XSFM supplemented with cytokines, they were able to maintain a high percentage of CD34+ hematopoietic progenitor cells.
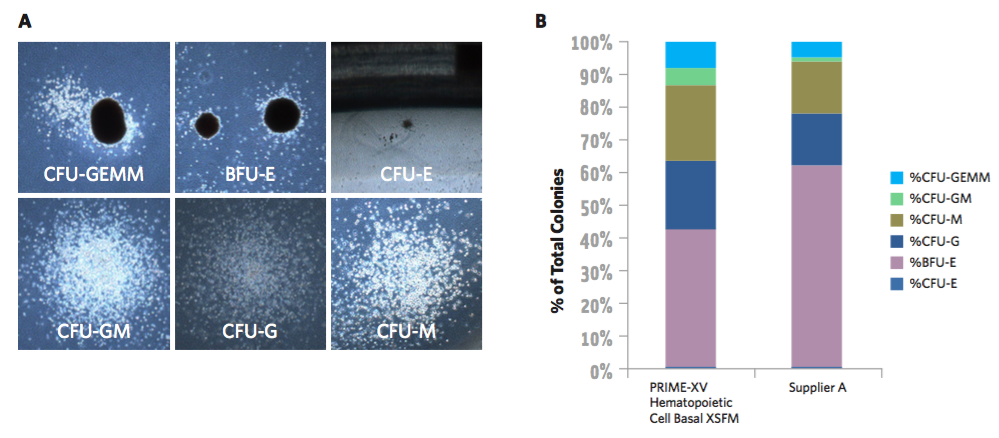
Maintains balanced lineage differentiation potential
Assessment of the differentiation potential of HSPCs is commonly conducted using a colony-forming unit (CFU) assay. CFU assays have been shown to be a strong independent predictor of successful engraftment and as such, CFU dose is now used as an important part of graft selection and often correlates with reconstitution of HSPCs following transplantation1,2. In figure 3, Irvine Scientific presents CFU assay data that demonstrates PRIME-XV Hematopoietic Cell Basal XSFM maintains differentiation potential and supports balanced distribution of lineage subtypes.
Manufactured to facilitate transition from research to clinic
Irvine Scientific has designed this medium to facilitate the progression cells or research work toward clinical application. The first step in this is to reduce the risk of adventitious agents, by making the media serum-free and xeno-free. Next it is important to provide traceability documentation including Certificates of Analysis, Certificates of Origin, and filing of a Drug Master File (DMF) with the US FDA to ease regulatory filings. It is also key, if the media will be used in Cell Therapy manufacturing, that the medium undergo extensive QA testing including functionality, sterility and endotoxin testing. Lastly, Irvine Scientific maintains strict control of raw materials and manufactures their cell culture products in cGMP compliant and FDA-regulated facilities, which provides the level of quality, consistency and reliability required when working in clinical applications. All of the regulatory considerations that have gone into the creation of PRIME-XV® Hematopoietic Cell Basal XSFM, smooth the path toward use of hematopoietic cells for Cell Therapy applications.
For further product information, please visit PRIME-XV Hematopoietic Cell Basal XSFM
For more information or to obtain samples, please contact Irvine Scientific: Tel: +1 949 261 7800; Email: getinfo@irvinesci.com
Footnotes
-
1. Page et al. (2011) Blood Marrow Transplant 17(9):1362-1374
-
2. Yoo et al. (2007) Bone Marrow Transplant 39(9):515-521