
Xeno-Free, what is it?
Cells are complex and intricate microscopic wonders that are now being recognized as having infinite potential as therapeutic agents to fight against devastating human disease. The Cell Therapy space is now rapidly growing in the pharmaceutical industry with accelerated efforts amongst the top biotech organizations to either acquire or internally develop and advance these agents through the developmental pipeline. Why is it then that a therapeutic approach of such high and innovative caliber would be produced in a system that is undefined, variable, and potentially hazardous? Fetal bovine serum (FBS), although historically used as the standard cell culture supplement to propagate cells ex vivo, comes with the burdens of these unfavorable characteristics and therefore it is apparent that enhanced definition of cell culture systems is necessary to sustain these novel pharmaceutical advancements.
Given the well-recognized issues with FBS use, it should come to no surprise that in recent years there has been a push to transition away from this variable substance and toward a higher degree of chemical definition in cell culture media for both research and manufacturing purposes. The majority of cell culture media optimized for Cell Therapy applications available in the market today is known as xeno-free. But what does this mean? Xeno, derived from the Greek word xenos, means “stranger” or “foreigner”1. Putting this in terms of media suitable for expansion of human stem or primary cells, xeno-free simply means a formulation only comprised of human-derived components and does not incorporate foreign species such as bovine or porcine.
Xeno-free media for multiple therapeutic cell types have been extensively marketed and subsequently utilized in countless manufacturing processes for clinical cell product. These media exhibit satisfactory ability to expand therapeutic cells that are phenotypically correct. However, a common misconception around the term “xeno-free” is that the issues with FBS can be circumvented and that a new level of chemical definition and regulatory compliance is guaranteed. Indeed, widespread confusion in the industry has been observed due to the inconsistencies of how the terms xeno-free, animal-free, and serum-free are used 2. In actuality, xeno-free cannot offer enhanced chemical definition or the dependability of a cell culture system. Undefined substances such as human serum and human platelet lysate (hPL), though considered to be xeno-free, are not equivalent to a chemically defined media both in terms of quality, potential variability and ultimately, performance.
The inconsistency of human serum as both a starting material as well as a final cell culture additive is well known. Specific mitigation steps, such as pooling large numbers of donors, are routinely undertaken in the manufacturing of human serum and human serum-derived components to circumvent these variability issues.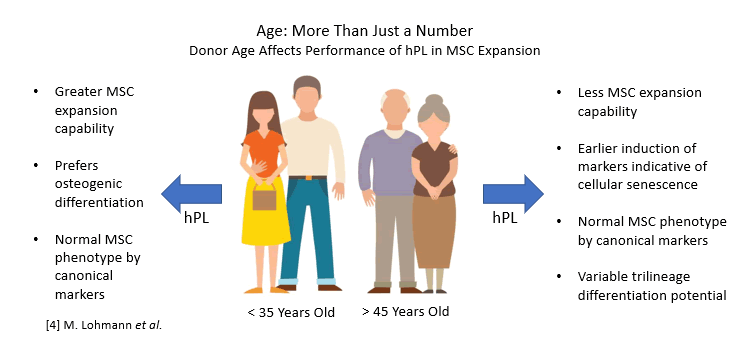 However, pooled serum can actually result in reduced bioactivity compared to individual donors depending on the availability of donors at any given time 3. It has also been shown that hPL derived from younger donors was associated with enhanced expansion of mesenchymal stem cells as well as more prominent osteogenic differentiation over aged donors 4.
However, pooled serum can actually result in reduced bioactivity compared to individual donors depending on the availability of donors at any given time 3. It has also been shown that hPL derived from younger donors was associated with enhanced expansion of mesenchymal stem cells as well as more prominent osteogenic differentiation over aged donors 4.
Further, media formulations that incorporate components purified from human serum, such as human serum albumin and transferrin, can potentially have variability issues. The manufacturing guidelines for human serum albumin only require it be 96% purity or greater, meaning 4% of this vital supplement contains unknown and potentially biologically active substances and factors that may interfere with the expansion, differentiation and functionality of the target cell line 5. If a medium contains a certain percentage of undefined human serum-derived contaminates, does the label of “serum-free” even still apply? Additionally, discrepancies in preparations of human serum albumin have been shown to result in significant posttranslational differences, potentially affecting the overall protein function 6. Given the relatively high incorporation rates of human serum albumin in cell culture media formulations, these undefined human serum-derived contaminates combined with the heterogeneity of the albumin can potentially cause unexpected outcomes in cell culture systems.
Finally, the inclusion of human serum or human serum-derived components in cell culture media for clinical manufacturing introduces the risk of adventitious agent contamination. Thus in order to lower, but not totally mitigate, the risk associated with the utilization of these regents, additional resources must be invested to certify there are no known pathogens associated with blood-derived components via virus-specific testing. While, to date, there have been no reported cases of virus transmission from human serum-derived components, such as albumin, there have been outbreaks associated with more complex serum-based products such as plasma protein fraction (PPF) 7. These findings indicate a differential safety profile within the xeno-free class of media, creating further confusion when blanket terminology is applied to different products with varying chemical definition on the market.
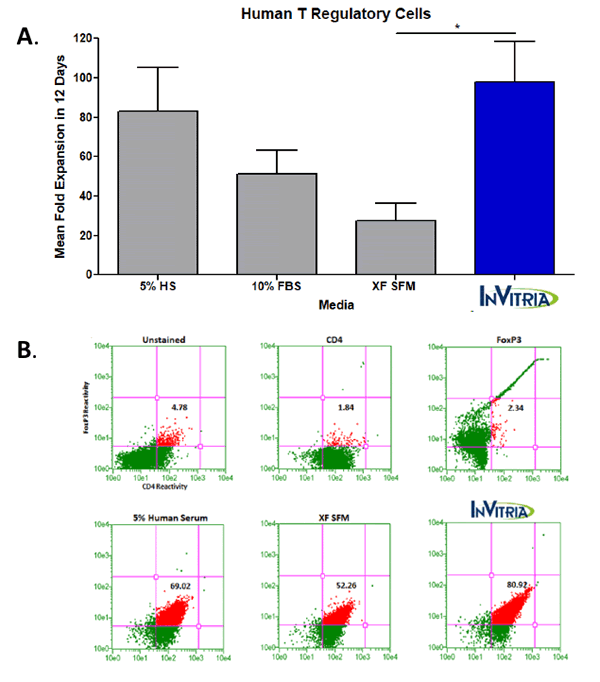
These potential safety and variability concerns in the xeno-free class of cell culture media has prompted the desire to enhance the quality and chemical definition of cell culture media beyond the current xeno-free standard. Blood-free, a new class of cell culture media, incorporates recombinant proteins with exceptional purity and biological activity that have been produced at commercial scale in the place of serum-derived proteins. Cellastim S, a recombinant human serum albumin, has demonstrated exceptional ability to support cell proliferation in multiple therapeutically relevant cell lines in vitro. Additionally, Optiferrin, a recombinant human transferrin, has been confirmed to be both structurally and functionally similar in its iron-binding capabilities and has been validated to perform as an effective replacement to serum transferrin in several bioprocessing applications 8, 9. Complete blood-free media formulations that are built around the highly pure Cellastim S, Optiferrin, and ITSE AF have demonstrated the ability to produce higher quality and more functional cells than those produced in serum and serum-derived component-containing media simply due to the fact that this “clean media” does not include potentially conflicting signaling to cells from competing serum-derived contaminates. Figure 1 demonstrates this point in human T Regulatory Cells, though multiple cell types have been evaluated to date.
 Thus, the movement to blood-free media systems is the subsequent step in leveraging the knowledge of cell culture media function derived from xeno-free media design while presenting a logical solution to circumvent the reproducibility and safety issues associated with native-derived proteins and serum fractions. Incorporation of these blood-free proteins ensures better chemical definition in cell culture media, something the xeno-free campaign failed to accomplish. This enhanced definition drives reproducibility, a deeper understanding of factors that result in desirable outcomes in cell culture, and fuels additional research and development, ultimately ensuring a safer therapeutic for the patients who are depending on them.
Thus, the movement to blood-free media systems is the subsequent step in leveraging the knowledge of cell culture media function derived from xeno-free media design while presenting a logical solution to circumvent the reproducibility and safety issues associated with native-derived proteins and serum fractions. Incorporation of these blood-free proteins ensures better chemical definition in cell culture media, something the xeno-free campaign failed to accomplish. This enhanced definition drives reproducibility, a deeper understanding of factors that result in desirable outcomes in cell culture, and fuels additional research and development, ultimately ensuring a safer therapeutic for the patients who are depending on them.
Footnotes
-
1. “xeno-
-
2. Definition of xeno- in US English by Oxford Dictionaries.” [Accessed: 24-Jan-2018].
-
3. Hemeda, B. Giebel, and W. Wagner, “Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells,” Cytotherapy, vol. 16, no. 2, pp. 170–180, Feb. 2014.
-
4. Arjmand, Perinatal Tissue-Derived Stem Cells: Alternative Sources of Fetal Stem Cells. Springer, 2016.
-
5. Lohmann et al., “Donor Age of Human Platelet Lysate Affects Proliferation and Differentiation of Mesenchymal Stem Cells,” PLOS ONE, vol. 7, no. 5, p. e37839, May 2012.
-
6. Desai, P. Rambhia, and A. Gishto, “Human embryonic stem cell cultivation: historical perspective and evolution of xeno-free culture systems,” Reprod. Biol. Endocrinol. RBE, vol. 13, Feb. 2015.
-
7. Bar-Or, R. Bar-Or, L. T. Rael, D. K. Gardner, D. S. Slone, and M. L. Craun, “Heterogeneity and oxidation status of commercial human albumin preparations in clinical use,” Crit. Care Med., vol. 33, no. 7, pp. 1638–1641, Jul. 2005.
-
8. L. Erstad, “Viral infectivity of albumin and plasma protein fraction,” Pharmacotherapy, vol. 16, no. 6, pp. 996–1001, Dec. 1996.
-
9. Zhang et al., “Expression, purification, and characterization of recombinant human transferrin from rice (Oryza sativa L.),” Protein Expr. Purif., vol. 74, no. 1, pp. 69–79, Nov. 2010.
-
10. N. Steere et al., “Biochemical and Structural Characterization of Recombinant Human Serum Transferrin from Rice (Oryza sativa L.),” J. Inorg. Biochem., vol. 116C, pp. 37–44, Nov. 2012.