Zap-SR Utility in Expanding Cells Critical in Virus Production
Since their appearance in the late 18th century, vaccines have had a profound impact on human and animal health and have simultaneously shaped the field of immunological research for years to come. The emergence of recombinant DNA technology and advances in cell culture processes have only fueled the expansion of these therapeutic viruses into new and exciting areas of gene, cell, and immunocancer therapies. Future development strategies will surely only continue to evolve for the production of novel lifesaving virus-based therapies and vaccines.
Currently, several vaccines and viral vectors are produced in mammalian cell-based production systems for both animal and human use. Unlike the advances in the other bio production cell systems such as CHO, current manufacturing of several vaccines or viral vectors depends on the use of fetal bovine serum (FBS) to support acceptable cell growth prior to infection and subsequent virus production. However, falling supply and rising prices of FBS may hinder the production/commercialization of emerging viral vectors and vaccines. Supplements that would stretch this dwindling serum supply and at the same time counter the rising prices of serum would be extremely beneficial.
Here, we have demonstrated the efficacy of a new supplement, Zap-SR, in propagating VERO cells in reduced serum conditions. VERO cells (ATCC) were maintained in EMEM supplemented with 2 mM Glutamine and 10% FBS for at least 2 passages prior to the experiment. To initiate the comparison, VERO cells were directly passaged into EMEM + 10% FBS or EMEM supplemented with either 2% FBS or 2% FBS with 8 mLs/liter complete media plus Zap-SR. Cells were subcultured approximately every 96 hrs for a total of 3 passages in 10 days. Cultures were propagated in triplicate.
With an 80% reduction in FBS, VERO cells showed slower doubling times and significantly lower numbers of cumulative population doublings when compared to cells expanded in 10% FBS (9.05 ± 0.59 versus 11.5 ± 1.01 cumulative doublings, respectively). However, the addition of Zap-SR efficiently rescued the doubling time and cumulative doublings to comparable levels of 10% FBS (10.66 ± 1.19).
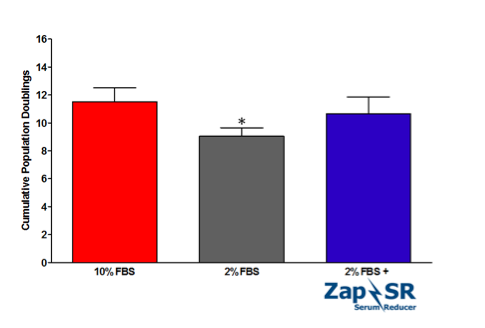
This proliferation data hints at the utility of Zap-SR in expanding cells that are relevant in vaccine and therapeutic virus production in reduced serum conditions. The incorporation of Zap-SR allows conservation of the dwindling serum supply and simultaneously creates cost savings for the end user. In addition, these encouraging results prompt the further investigation of Zap-SR efficacy in expanding VERO cells in more relevant culture systems such as spinner culture in addition to testing Zap-SR in other cell lines that are commonly used in virus production.