The Number of FDA Drug Approvals for the 2012 Fiscal Year Remains High
In a recent report issued by the Food and Drug Administration (FDA) “Fiscal Year 2012 Innovative Drug Approvals,” the agency highlighted 35 new drug approvals. This marks the second year in a row where FDA has issued 35 approvals and continues the trend of more annual approvals than in years past. In addition, FDA approved 77 percent of these drugs on the first review cycle without the need for additional information. Of the 35 drugs, 32 were also approved in other countries, and in these cases, FDA was the first to approve 75 percent of the time.
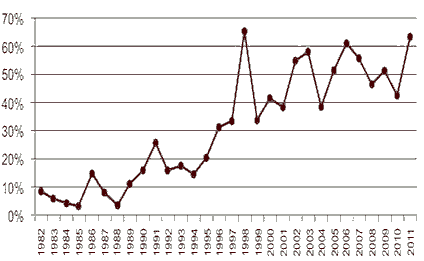
FDA has attributed higher approval rates to the use of FDA programs including Fast Track, Priority Review, and Accelerated Approval. FDA has also been successful in improving the approval process by streamlining reviews, increasing communication with drug companies during the review process, and meeting review deadlines. The FDA continues to lead the world in the first introduction of new active substances. According to the report “Over the past decade, roughly half of the new active substances launched anywhere on the world market were approved in the United States (see Figure 1). This year’s approvals contained ground breaking drugs in cancer and cystic fibrosis and also some firsts including the United States’ first approved drug from cord blood and first approved drug manufactured using genetically engineered plant cells.
Genentech’s Perjeta (pertuzumab) was approved in June for treatment of late-stage breast cancer. Perjeta is a recombinant humanized monoclonal antibody manufactured in chinese hamster ovary (CHO) cells. A study presented last week at the San Antonio Breast Cancer Symposium, found that Perjeta when combined with Herceptin and chemotherapy extended the lives of women with HER2-positive breast cancer. The combination of Perjeta, Herceptin, and chemotherapy reduced the risk of death by 34 percent when compared with placebo, Herceptin, and chemotherapy. Approval of Perjeta offers hope to many with HER2-positive breast cancer because this form of breast cancer is very aggressive and currently incurable.
Another highlight was the approval of the first cord blood product in the United States in November 2011, HEMACORD (hematopoietic progenitor cells, cord (HPC-C)). HEMACORD consists of hematopoietic progenitor cells, monocytes, lymphocytes, and granulocytes derived from human cord blood for the treatment of blood disorders. The progenitor cells are infused into patients where they migrate to the bone marrow. Once there, cells divide and grow and then move back into the bloodstream where they help build new blood cells. It is indicated for use in patients with a range of blood disorders that could include the treatment of patients with certain blood cancers and some inherited metabolic and immune system disorders.
The FDA also approved the first drug produced in a genetically engineered plant cell. Elelyso (taliglucerase alfa) is manufactured using genetically modified carrot root cells. The drug approved in May 2012 for treatment of Gaucher Disease, competes with Genzyme’s Cerezyme and Shire’s Vpriv, both manufactured in mammalian cell culture. Pfizer purchased global rights to Elelyso, (outside of Israel), from Protalix. Protalix developed the plant based expression system and has promised to avoid the supply interruptions that have caused problems for Genzyme in the past. Elelyso provides another option for patients with Gaucher Disease, a rare genetic disease that causes an accumulation of glucocerebroside to build up in the spleen, liver, bones and sometimes the brain and prevents organs from functioning correctly.