A New Chemically-defined Medium for use with Suspension-adapted Avian Cells in Vaccine Manufacturing
Much of current cell-based vaccine manufacturing is done in cell lines like Vero (African green monkey kidney), MDCK (Madin-Darby canine kidney) or CEF (chicken embryo fibroblast) cells. These cell lines are adherent cells that require a surface to attach to in order to proliferate and produce viruses. Production scale-up with adherent cells is generally more challenging than with suspension cell cultures due to the surface area requirements for adherent cells.
For adherent cells to be used in large-scale vaccine manufacturing, the cells must be either attached to microcarriers for culture in traditional bioreactors, or cultured in fixed-bed bioreactors that have a surface for adherence built in. Adherent cells can also grow in cell stacks or roller bottles in smaller scale. Large-scale manufacturing almost always requires the use of microcarriers, but the use of microcarriers often complicates manufacturing processes due to challenges with shear and separating the cells from the microcarriers themselves. Therefore, many efforts have been made to adapt adherent cell lines in vaccine manufacturing into suspension mode, where scale-up can be achieved by simply employing larger volume bioreactors. However, the lower viral titers typically resulting from these efforts are unsuitable for use in large scale manufacturing.
New Cell Lines for Viral Vaccine Manufacturing
In response to the challenges of adherent cells for vaccine manufacturing, researchers have explored the use of other cell lines that are more amenable to suspension culture. Examples of such cell lines include EB66®, PER.C6®, and CAP®.
The EB66 avian cell line, developed by Valneva, Inc. from duck embryonic stem cells, has been investigated for use in virus production with very exciting results (Madeline et al 2015, Léon et al. 2016). EB66 cells can be grown in serum-free suspension culture at high cell density, enabling much easier and more efficient scale-up than with attachment cells. More importantly, virus production levels using these cells have been comparable to or better than those obtained using attachment cells.
Interest in exploring new cell lines for vaccine manufacturing, in particular the EB66 cell line, has been increasing. In June 2016, Valneva announced a new R&D collaboration with GlaxoSmithKline (GSK) for pre-pandemic influenza vaccine based on the EB66 cell line. Prior to that, the first human vaccine using the EB66 technology received marketing approval in 2014, with the first veterinary vaccine receiving approval in 2012.
In support of this type of cell line, GE Healthcare recently launched HyClone™ CDM4Avian Medium, a chemically-defined medium for both cell growth and virus production in the EB66 avian cell line. The development of this new medium was highlighted in a poster titled, “Development of a chemically defined medium for virus vaccine production in a duck suspension cell line.” The poster walks through the steps of the media development process and the results.
Poster Highlights
In the poster, the authors explain that typical viral production in EB66 cells is biphasic, meaning that the culture requires separate media for the cell proliferation phase as well as the virus production phase. In contrast, a monophasic process in this instance means that the same medium is used to support both cell expansion and virus production (Figure 1).
Figure 1:
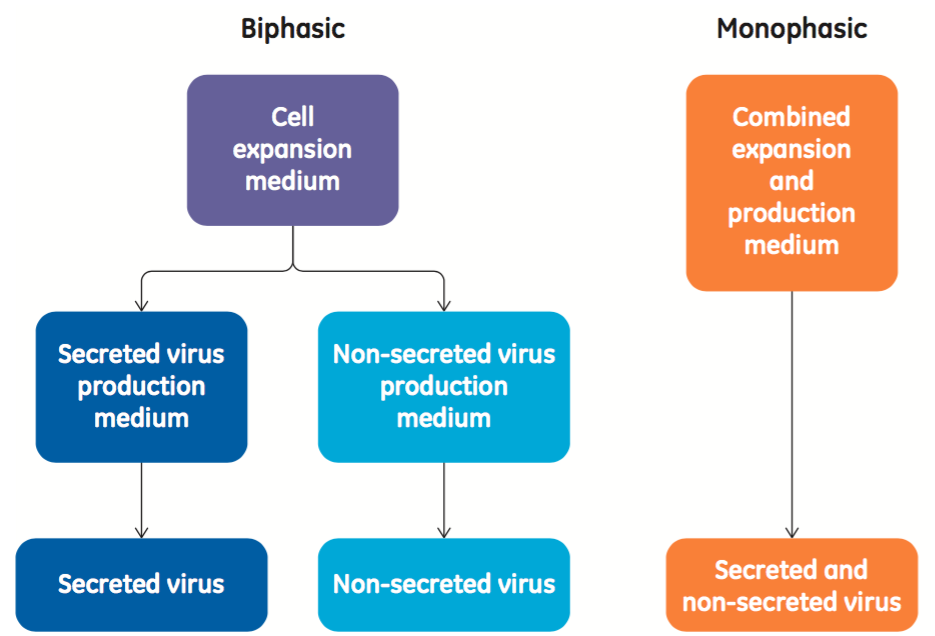
A biphasic process includes two different basal medium formulations, with several separate additives or feeds required that are serum-free but not chemically-defined. Thus, for ease of use, it is desirable to have a monophasic process, and for consistency and regulatory purposes, it is desirable to have a chemically-defined medium. When developing the new medium, GE collaborated with Valneva and identified desirable performance characteristics for the new medium. These included:
- Single medium supporting both cell expansion and virus production
- Maintenance of cell line stability for multiple passages, potentially increasing the size of the virus production window
- Support of high cell density and viability
- Maintenance of short, passage-consistent population doubling times (< 20 h)
- Support of process scaling by yielding similar growth and virus production from shake flask to bioreactor scale
- Support of high productivity of a variety of viral types
- Support the use of cryopreservation and cryorecovery processes.
Media Development
To begin development of the new medium, a growth-only screen of 20 different media formulations, mixtures and/or fortifications of existing media was conducted. The goal was to identify the most promising formulations to begin with. Factors evaluated included good cell growth and viability as well as consistent cell morphology and aggregation level. Cell counts and viability were determined with a Vi-CELL™ counter (Beckman Coulter).
The screened formulations were then evaluated for viral production of both secreted and non-secreted viruses; namely, paramyxovirus, poxvirus, and alphavirus. Viral titer was determined by standard tissue culture infective dose 50% (TCID50) methods.
Since the ability to scale up these cultures quickly and efficiently is an advantage for this cell line, scale up capability is a critical factor. As such, scale up to bioreactor culture was also evaluated and the bioreactors used for the study included single-use Xcellerex™ XDR-10 bioreactor (10 L) and WAVE™ Bioreactor 20 (10 L) systems.
Study Results
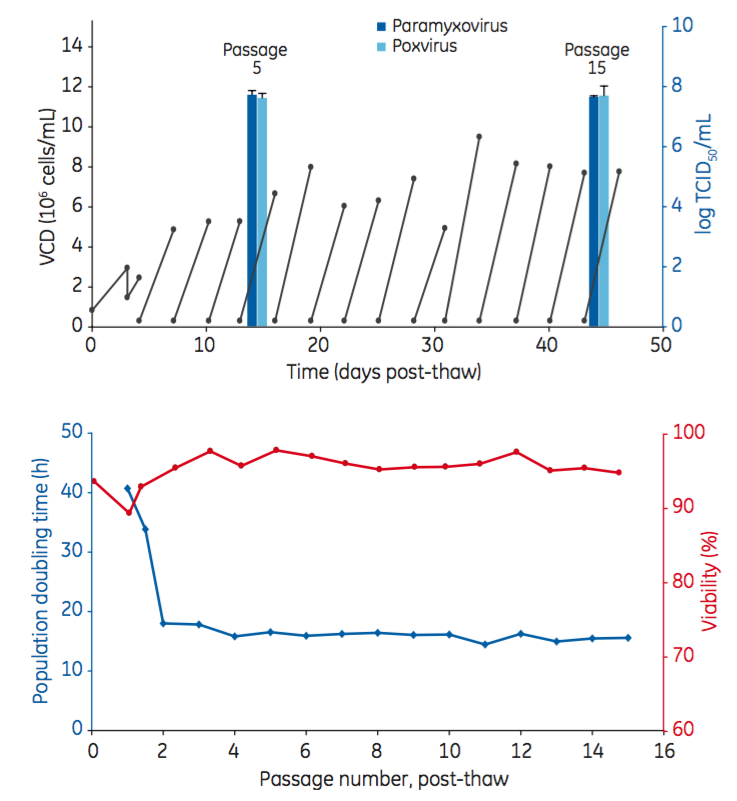
The results of the study demonstrated that EB66 cells were able to be directly adapted to the new HyClone CDM4Avian medium. In addition, equivalent productivity was demonstrated ten passages apart indicating stable performance (Figure 2). Cell surface marker analysis confirmed no detectable drift over these ten passages.
Figure 2:
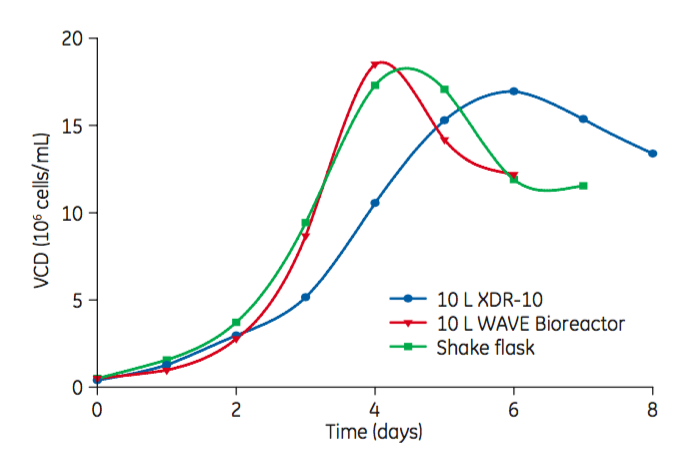
Scalability was tested from shake flask to bioreactor. While growth in the XDR-10 bioreactor system was slightly slower, all three cultures yielded similar peak viable cell densities (Figure 3).
Figure 3:
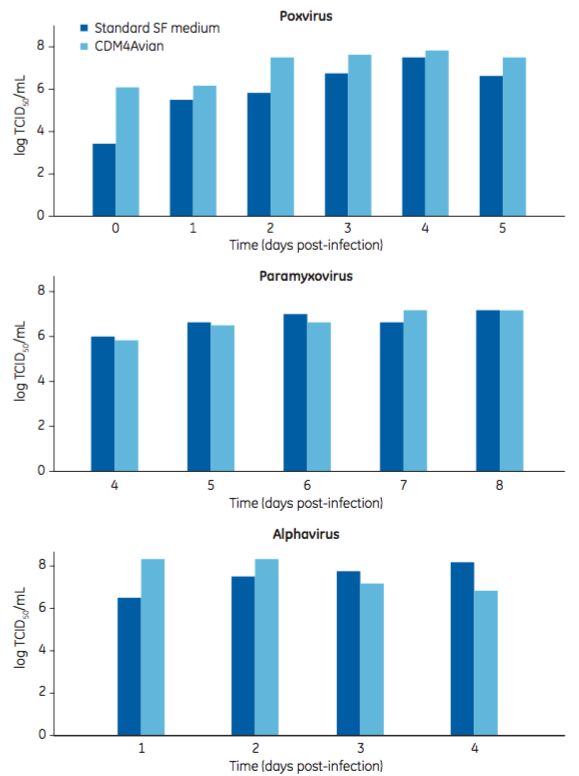
Virus production using the monophasic CDM4Avian medium process was as good as or better than the biphasic process (Figure 4). In some instances, titer was significantly improved. For instance, virus productivity of a B strain of orthomyxovirus was better in the CDM4Avian medium, with a hemagglutinin concentration four times the level of that obtained in the reference process (data not shown).
Figure 4:
Summary
In viral vaccine manufacturing, the development of new suspension adapted cell lines circumventing the challenges of adherent cell culture scale up is increasing. The EB66 suspension-adapted cell line has demonstrated the ability to quickly and efficiently scale up for cell growth and virus production. GE Healthcare, in collaboration with Valneva, has developed a chemically defined medium for the suspension cell culture of the EB66 cell line. The cells adapted easily to the new HyClone CDM4Avian Medium and demonstrated excellent stability of cell phenotype, morphology, densities, and population doubling times after thirty passages. Embryonic cell surface markers also remained consistent after thirty passages, while viral productivity remained constant for at least the fifteen passages that it was measured. In addition, GE Healthcare has reported that the CDM4Avian medium monophasic (single medium) approach has been investigated with several additional viruses with encouraging results.
To learn more about HyClone CDM4Avian Medium, please visit GE Healthcare.
Please click on the poster to read the full study: