Synergizing Transient and Stable Protein Expression for Accelerated Biotherapeutic Development
Reducing drug development timelines and cost is a goal that we hear repeatedly in the industry. One area where timing is crucial is in reaching a go/no go decision on a therapeutic candidate and efficient production scale up once a go decision has been reached.
Early in development, small amounts of protein are needed to conduct preliminary studies that inform a go/no go decision. Protein can either be generated using transient production or through stable cell line generation. Most often companies choose to use transient transfection in either HEK or CHO cells to produce small quantities of protein because it is faster and more cost effective than developing a cell line. Having access to preclinical material faster and with less cost can greatly impact the overall drug development timeline. Then if the product progresses further in development, a stable cell line will be generated for larger scale manufacturing.
However, the timing of when to generate the stable cell line can be tricky. Stable cell lines are labor intensive and costly to develop. If companies develop one too early, the product may still fail preliminary studies and the time and money would be wasted. If companies develop the stable cell line too late, they may run into manufacturing, quality and timing delays.
After considering the challenges that exist in this model and the goal of reducing timelines and cost, what if companies could:
- Easily and cost effectively generate grams of protein using the host cell of choice earlier in development?
- Generate protein using transfection while at the same time creating a stable cell line?
I was excited to see that these questions and corresponding solutions were the subject of a recent webinar, “Synergizing Transient and Stable Protein Expression for Accelerated Biotherapeutic Development,” presented by Krista Steger, PhD Scientist, MaxCyte. Dr. Steger proposes solutions for both generating more protein earlier using the host cell line of choice and also developing a stable cell line in parallel using MaxCyte’s scalable electroporation technology. In the webinar, she presents data to support that transient protein generated using MaxCyte’s Flow Electroporation Technology is consistent with material generated via stable cell lines in terms of protein quality and glycosylation profiles. She also presents data to show that stable cell lines generated using the MaxCyte system are highly stable with consistent protein quality and glycosylation.
Generating Grams of Protein Earlier in Development – MaxCyte Flow Electroporation Technology for Transient Protein Expression
Technology
Dr. Steger begins with an explanation of MaxCyte’s flow electroporation technology. MaxCyte has developed an efficient and scalable transfection system based on electroporation. Electroporation involves the application of an electric field to cell suspensions, causing the cell membrane to become transiently permeable, which then encourages the entry of external materials into the cells. By utilizing the principles of electroporation, MaxCyte has developed a delivery platform that permits transfection of a wide range of cells with DNA, RNA, proteins or cell lysates and has demonstrated typical transfection efficiencies and cell viabilities at greater than 90% even for more difficult to transfect cells such as CHO cells. Another advantage of using electroporation is that it does not require specialized constructs, engineered cells, media additives or chemical reagents. The high performance of MaxCyte electroporation produces CHO based protein titers at > 2.7 g/liter with minimal optimization. This scalable system is able to transfect from 5×105 cells in seconds using small-scale, static electroporation up to 2×1011 cells in less than 30 minutes using flow electroporation.
Delivery Platform
MaxCyte systems are supplied with pre-loaded electroporation protocols optimized for a wide range of cell types, thereby simplifying assay development and maximizing performance and reproducibility. Identical electroporation parameters are used for small and large-scale electroporation enabling streamlined scale up to large volume transfections. MaxCyte offers the MaxCyte STX and the MaxCyte VLX to address both small and very large scale production requirements. Scale up from the STX to the VLX requires no further optimization. Results were presented showing that transfection using the MaxCyte STX and the MaxCyte VLX are consistent thus offering a streamlined scale up solution.
Transfection Efficiency
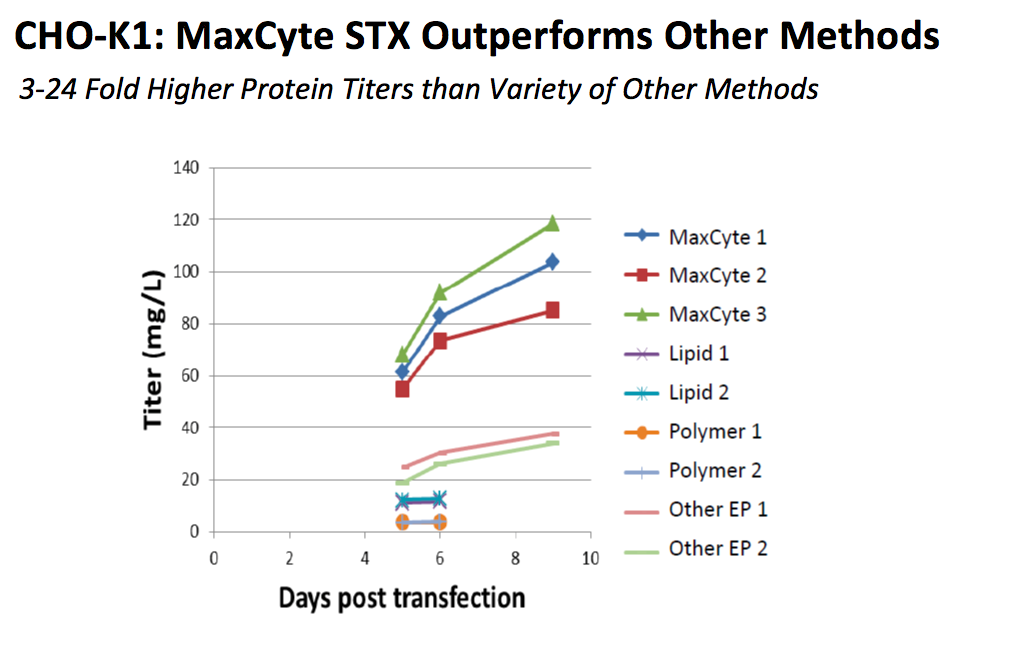
The MaxCyte electroporation-based platform is flexible and able to efficiently transfect a variety of CHO cell lines including CHO-S, CHO-K1, CHO EBNA, CHO-K1SV, and CHOZN®. Dr. Steger presented data to demonstrate how the MaxCyte transfection system compares to other methods of transfection in CHO K-1 cells (Figure 1).
Figure 1:
High Productivity
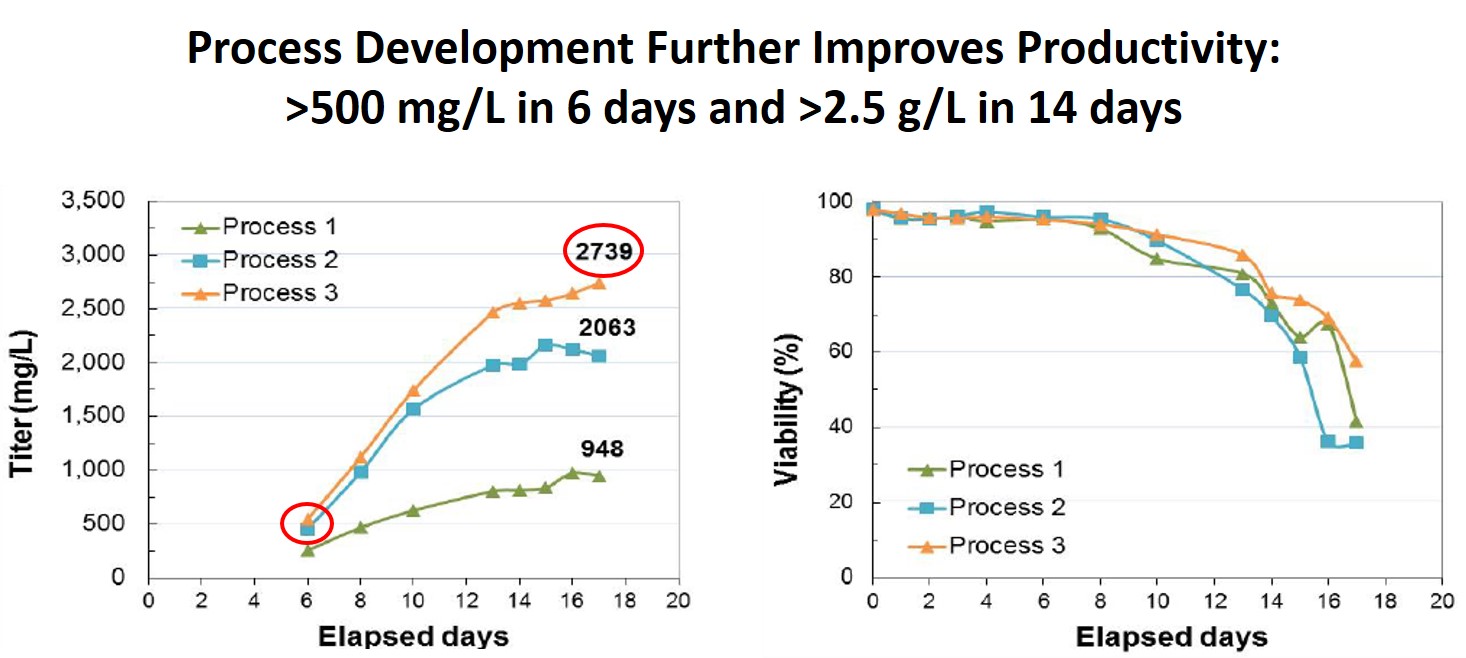
Using MaxCyte’s flow electroporation system, CHO cells can produce secreted antibody titers over 2.7 g/L with minimal optimization of post transfection culture conditions (Figure 2). Commercially available media and feeds were used in the study. With these high titers and MaxCyte’s scalability of up to 200 billion CHO cells in under 30 minutes, production of multiple grams of quality antibody can be achieved within 2 weeks of a single transient transfection. Thus enabling the production of grams of protein earlier in product development. Of note, titers of 500 mg/L were observed 6 days post electroporation, satisfying the needs of those researchers focused on the speed to production rather than maximum protein quantity.
Figure 2:
Consistent Quality and Glycosylation
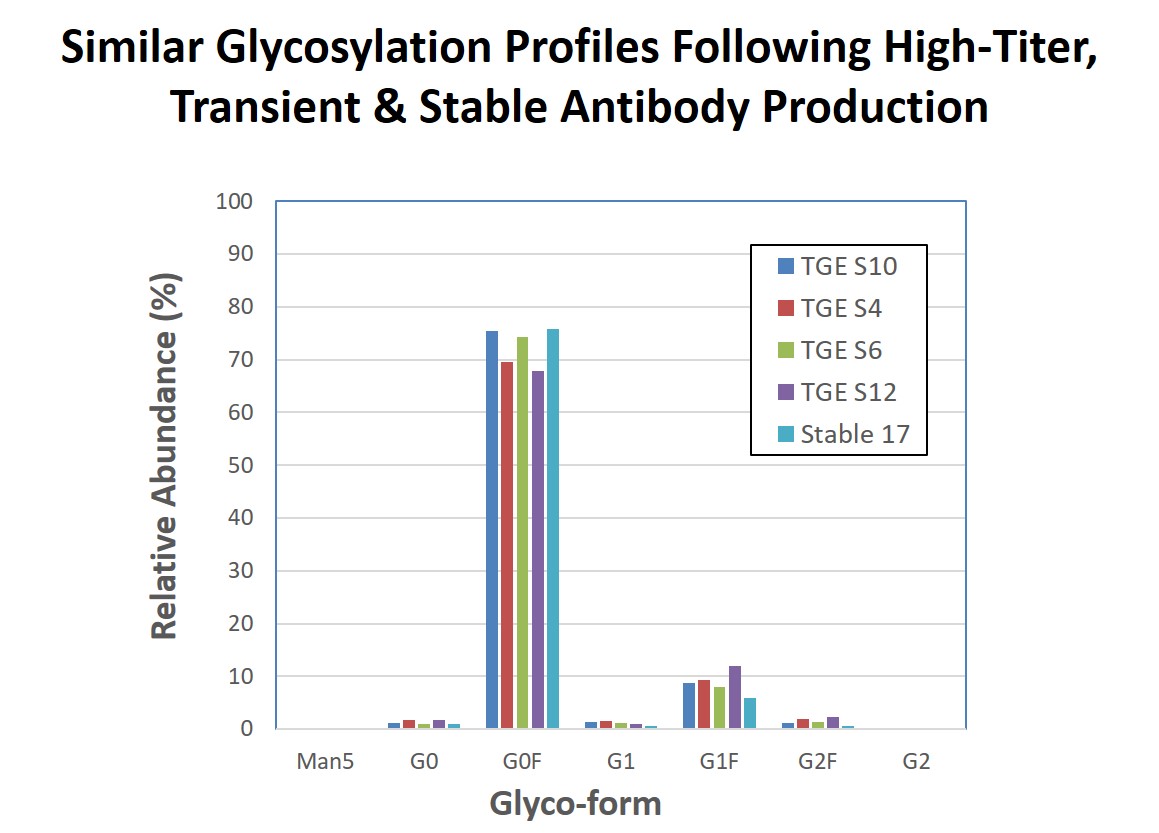
Dr. Steger then presented data on how MaxCyte transiently generated proteins compare to proteins generated using stable cell lines. For biotherapeutic development to fully benefit from transient production, the transiently produced candidate must accurately represents the product that will ultimately be manufactured via a stable cell line. Thus characteristics, such as protein quality and the glycosylation profile must be similar between the transiently produced material and stably produced material. Figures 3 and 4 demonstrate that antibodies produced via four electroporation runs are similar in both quality and glycosylation to antibody produced by a stable cell line that was generated following MaxCyte electroporation.
Figure 3:
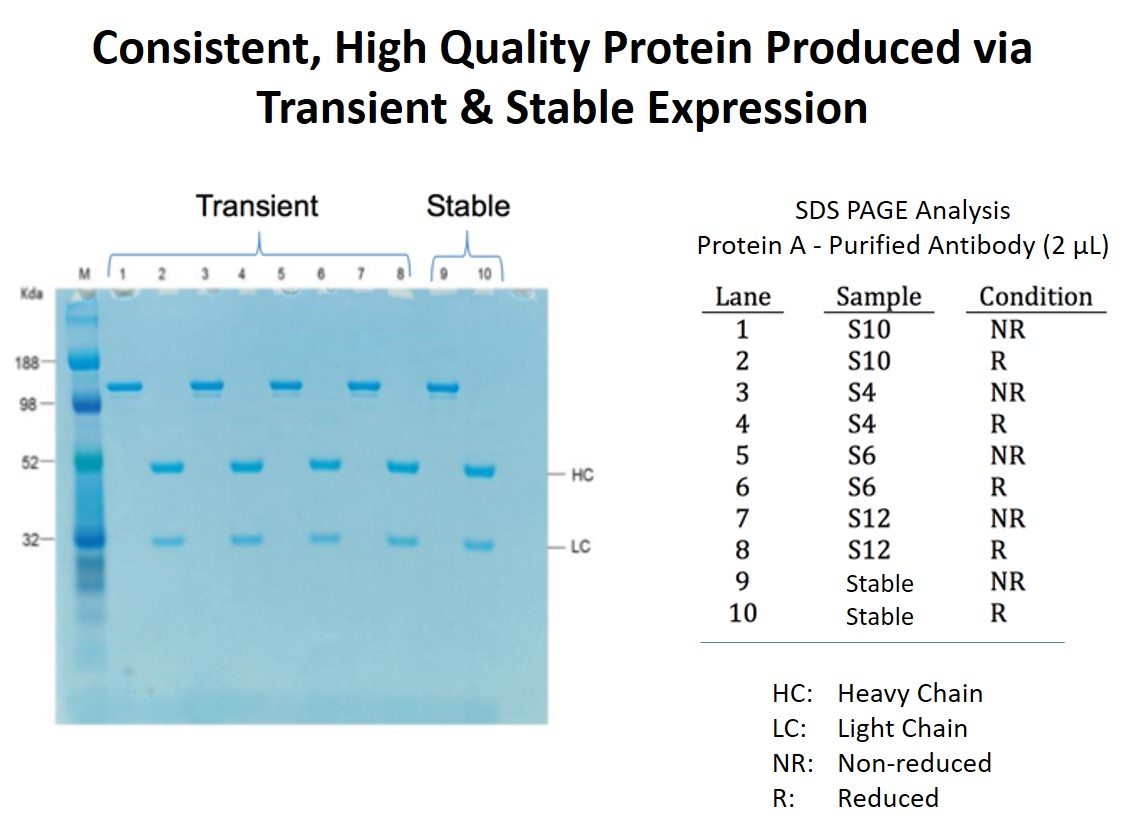
Figure 4:
Developing a Stable Cell Line in Parallel
After discussing the benefits of using MaxCyte’s electroporation for transient production, Dr. Steger presented data demonstrating the rapid creation of stable pools and high-yield stable cell lines. Data included cell line productivity, protein quality and glycosylation profiles over 60 days.
Reduced timeline for stable pool generation and stable cell line generation
MaxCyte electroporation shortens timelines of stable pool and cell line generation. Their high performance system produces faster cell recovery post electroporation compared with alternative transfection methods.. This faster recovery permits strong selection pressure to be applied.
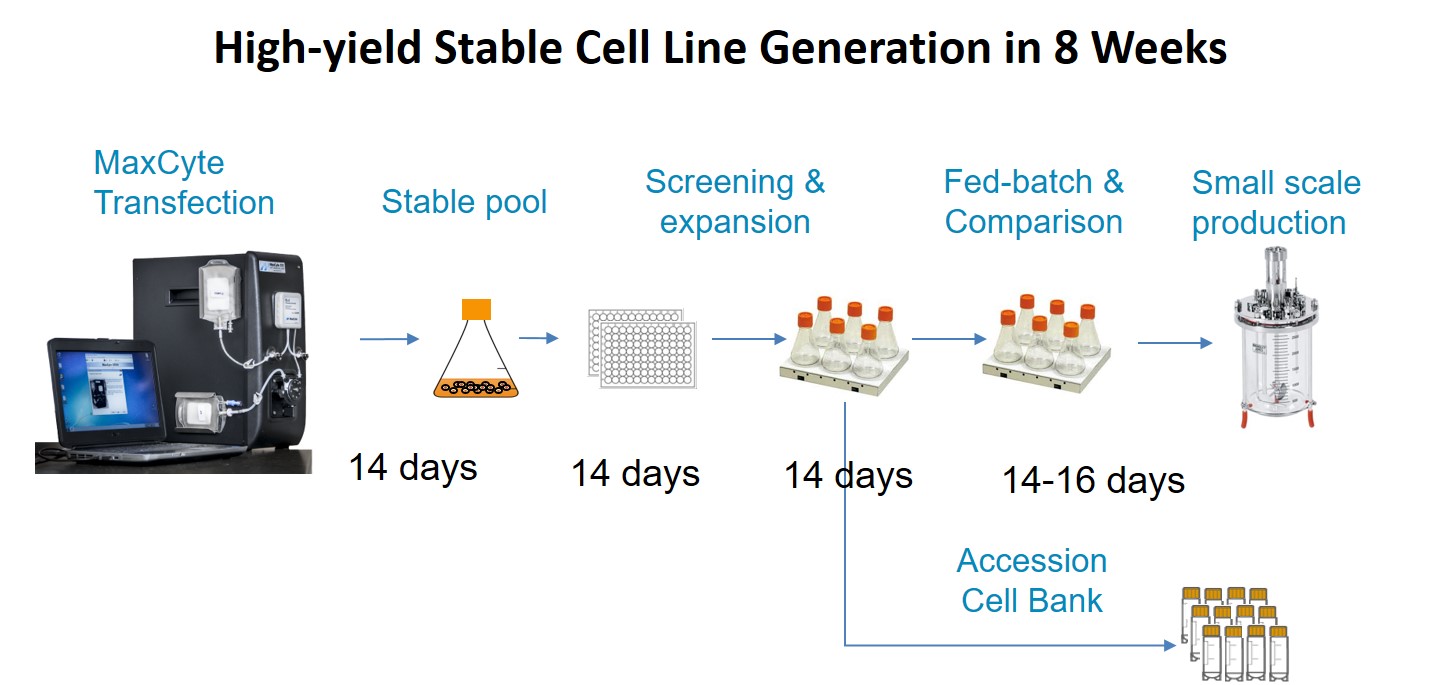
Due to the high CHO cell viability post electroporation (Figure 5), it is possible to generate stable pools 8 days faster for both an antibody and Fc fusion protein compared with a chemical transfection method. Additionally, stable single-cell clones were generated within 8 weeks with yields >5.7 g/L. Dr. Steger shared that they screened less than 500 clones to identify a high-yield stable cell line (Figure 6). Figure 7 provides an outline of the entire process.
Figure 5:
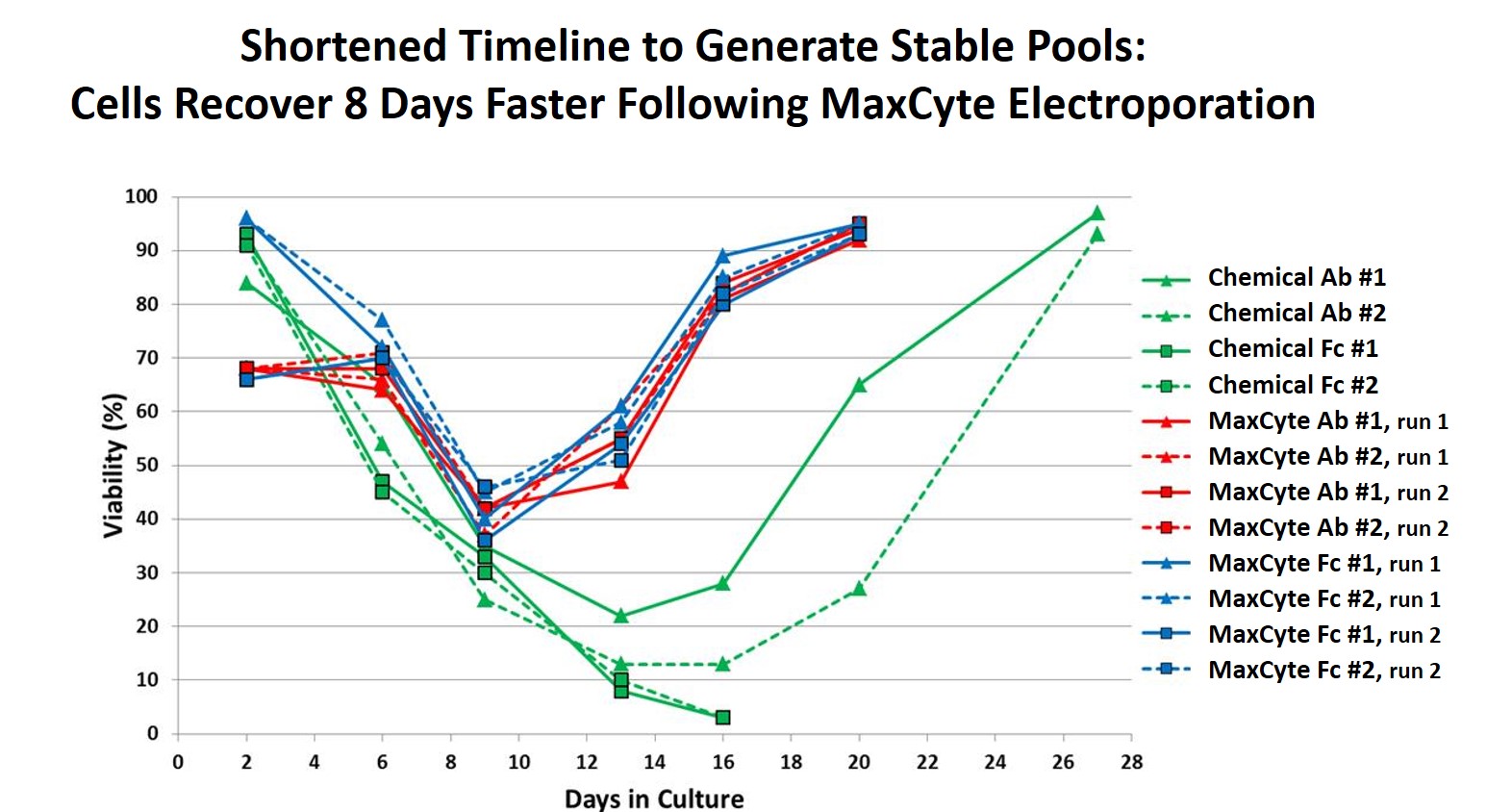
Figure 6:
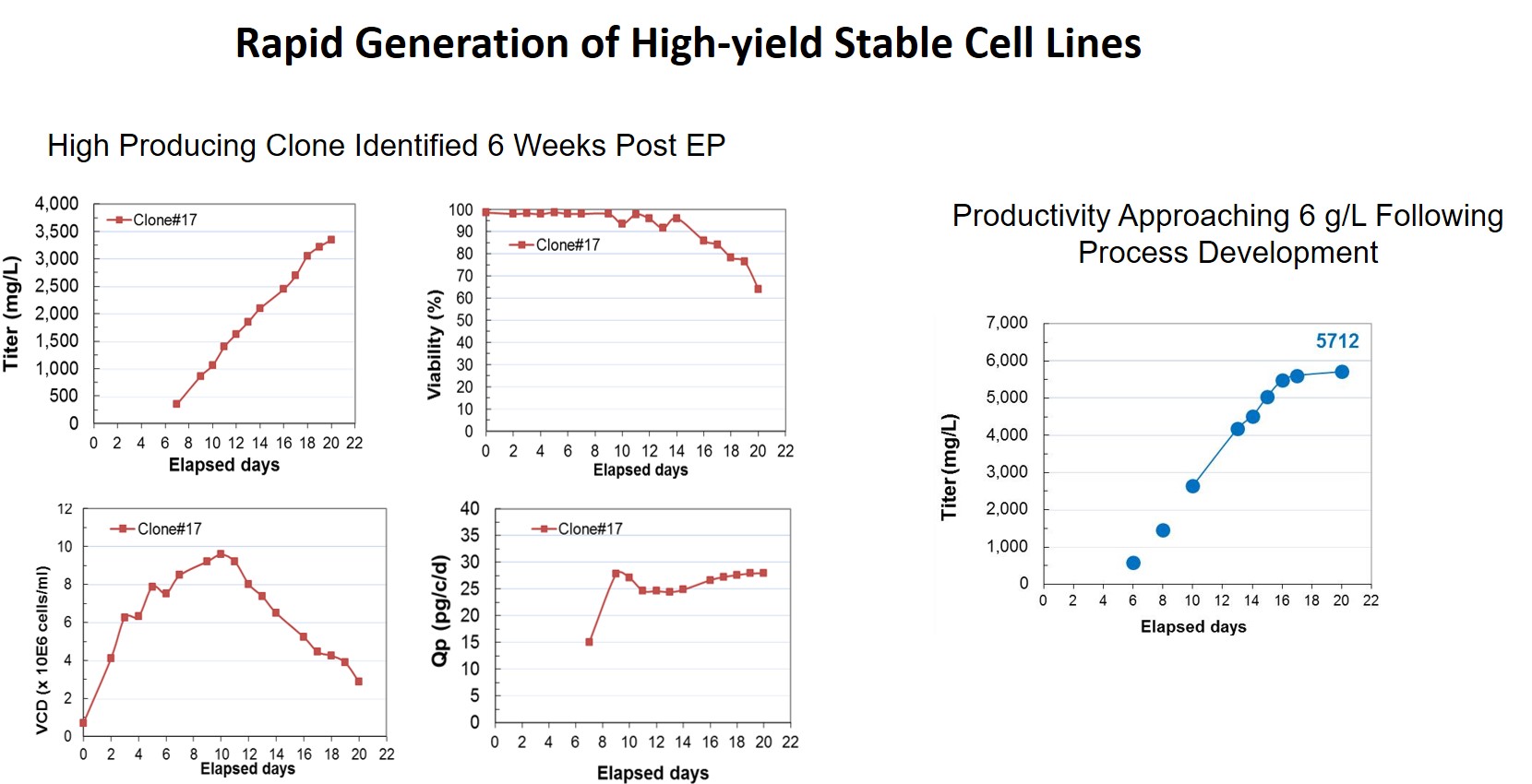
Figure 7:
Quality and stability of clones
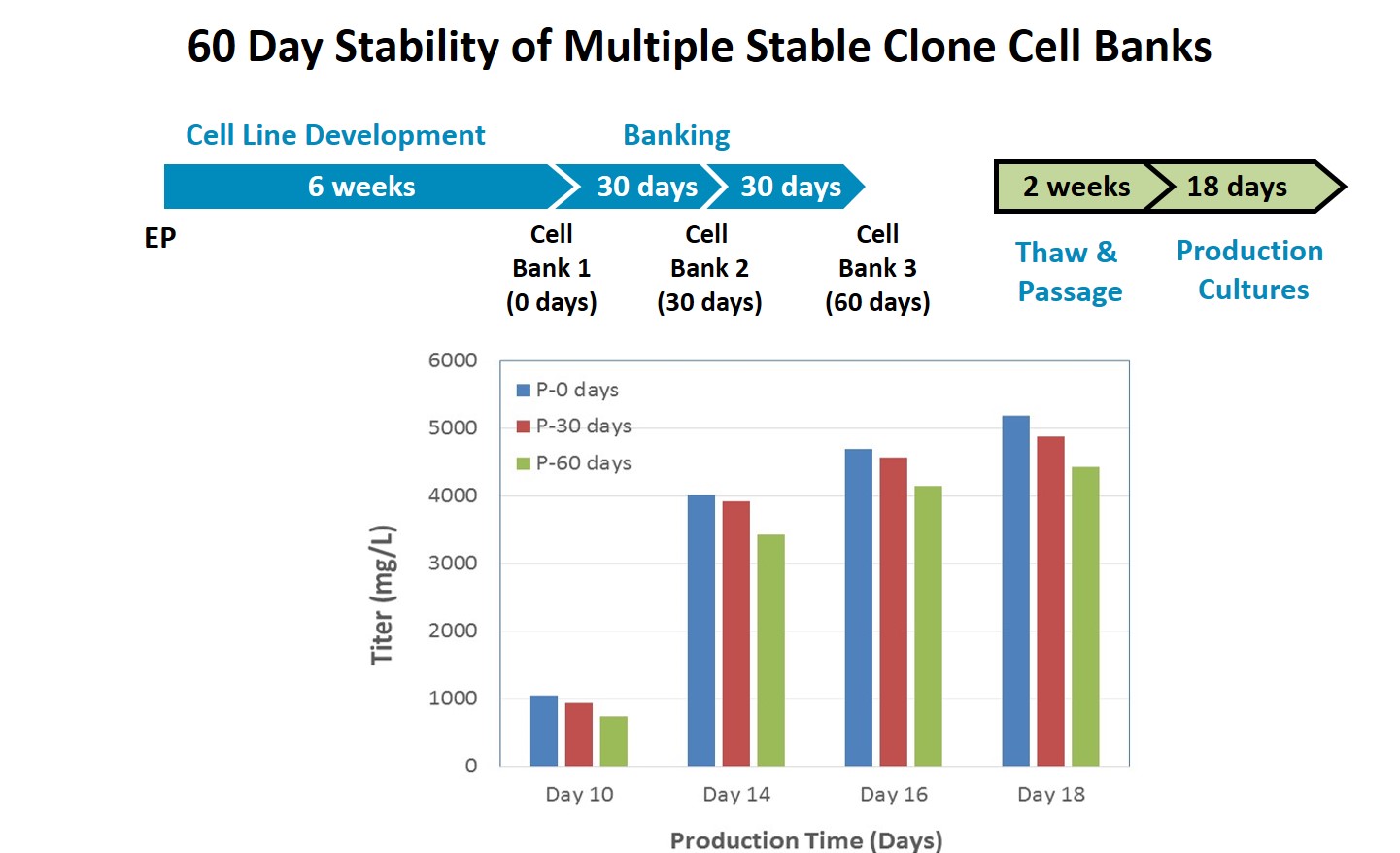
Dr. Steger went on to present data demonstrating the stability of the generated cell lines. To prove clone robustness and stability, she presented the results of a 60 day clone stability study. Data showed less than 15% loss in productivity detected between 1st, 2nd, and 3rd cell banks over the 60 days (Figure 8).
Figure 8:
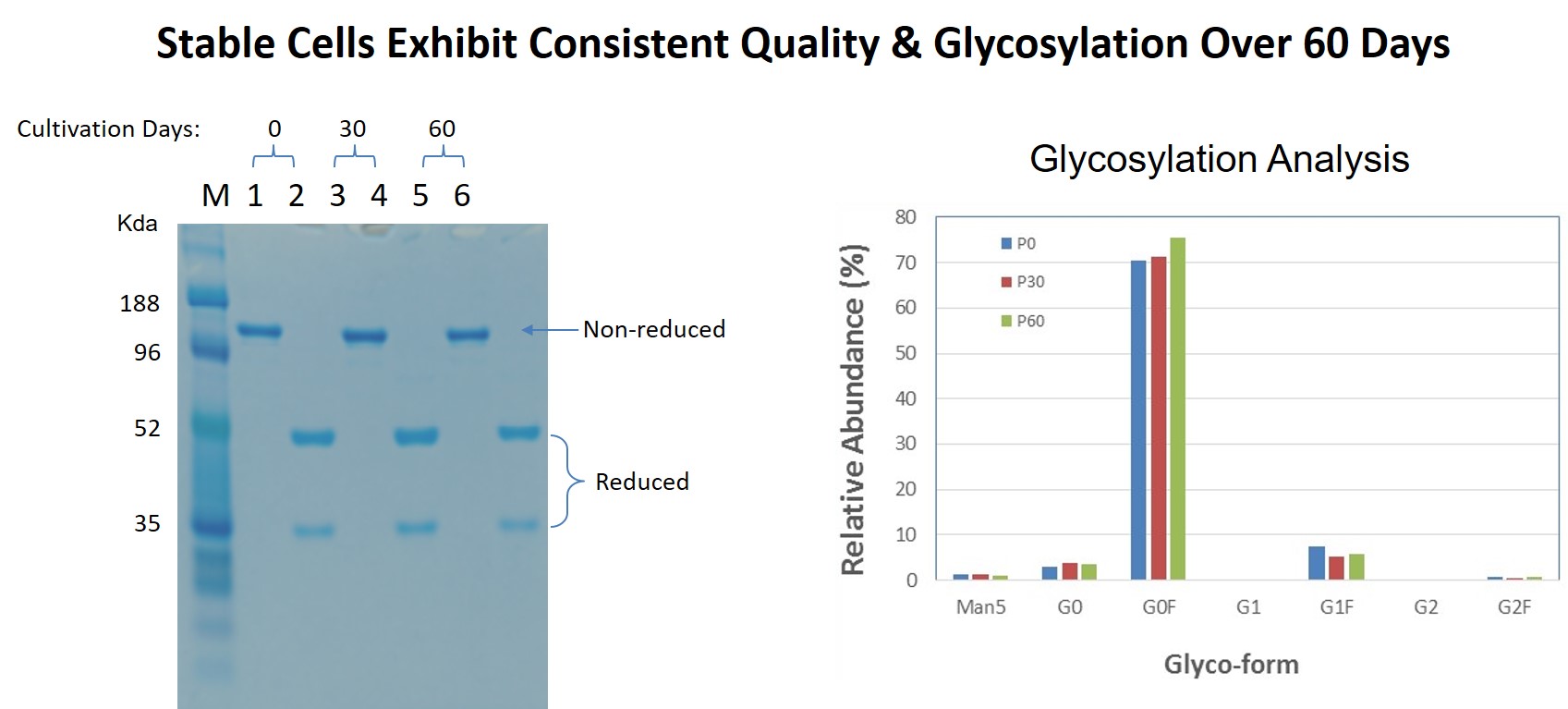
They also studied quality and glycosylation in the 60 day study and found that quality and glycosylation patterns were also maintained (Figure 9).
Figure 9:
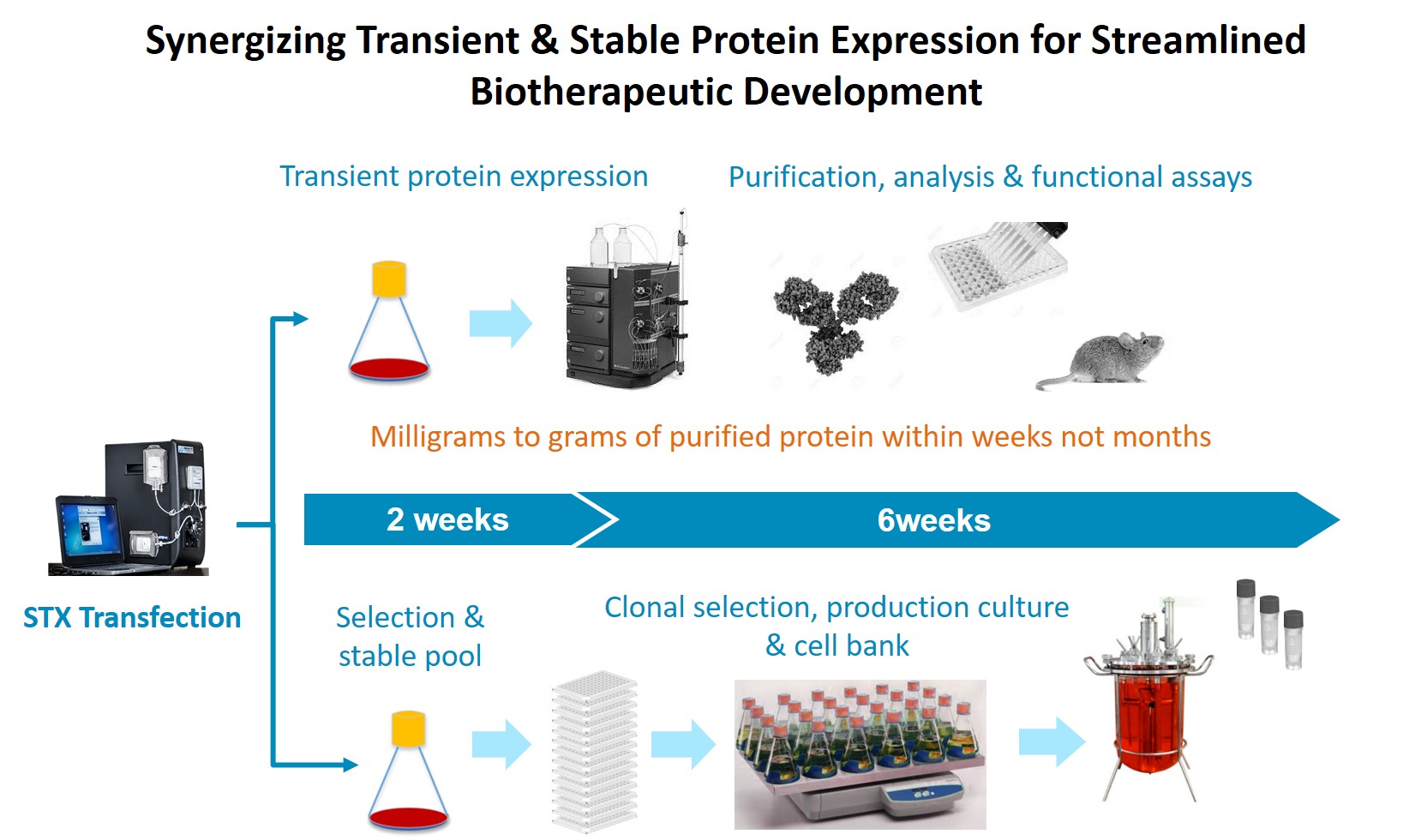
Lastly Dr. Steger presented a figure representing operation of the fully synergized protein expression platform with parallel transient production and stable cell line generation (Figure 10).
Figure 10:
Summary
In closing, Dr. Steger provided an overview of the benefits of using MaxCyte’s Scalable, electroporation-based delivery platform. These included:
- Working with the appropriate cell type, maximizing the relevance of the data produced
- Capability of producing a wide range of molecules (antibodies, bispecifics, fragments …)
- Producing sufficient material to drive development in weeks instead of months
- Improved productivity via higher efficiency and cell viability post transfection
- Shortened timelines to generating stable pools and clones
- Ability to parallel track transient production and stable cell line generation
- Accelerating the development of biotherapeutics while reducing costs
For further information about MaxCyte’s Scalable Electroporation, please see:
Webinar – Synergizing Transient and Stable Protein Expression for Accelerated Biotherapeutic Development
Previous Articles –
- Poster: Flow Electroporation Provides a Highly Efficient, Flexible and Scalable Transient Protein Expression System
- Electroporation-based Transfection Demonstrates Consistent Antibody Quality and Glycosylation Patterns for Biotherapeutic Product Development
You can also visit MaxCyte during BPI West at Booth #411