Efficient Large-Scale Expansion of MSCs for Translational Medicine and Research Use
As part of our ongoing conference coverage of ISSCR 2017, we like to feature posters from the event. One poster covered the very important topic of large-scale expansion of mesenchymal stem cells (MSCs) for both research and translational medicine. The poster, “Expansion of MSCs for Translational Medicine using MSC NutriStem® Basal Medium and PLTMax® Human Platelet Lysate,” was jointly presented by Biological Industries USA and Mill Creek Life Sciences, and highlighted at the Biological Industries booth. In the poster, authors describe the importance of successful large-scale expansion of MSCs and present data on a culture protocol that has generated very successful results.
Why is large-scale expansion of MSCs important?
Successful expansion of MSCs is important due to the popularity of the cell type in Cell Therapy applications. There are over 700 current or concluded clinical trials using MSCs worldwide, with 150 trials taking place in the United States and Canada.
MSCs are a popular choice for Cell Therapy applications primarily due to:
- Wide accessibility from various tissue types
- Cells’ ready expansion in culture
- Capacity for multipotent differentiation in vivo
- Biological properties that stimulate recovery of inflamed or injured tissues and organs and/or suppression of immune-related diseases
Important MSC Cell Therapy culture considerations
Historically FBS has been used to culture MSCs, but as these cells are increasingly being used in Cell Therapy applications, there has been an interest in finding animal-free supplement alternatives. However, when these cells are used in therapeutic applications, quality and safety are a top priority. In addition, they must be expanded to cell numbers that can meet the therapeutic cell dosage levels. This requires that media and supplements must meet high quality, safety and performance standards.
Successful large-scale expansion
In an effort to meet these needs, Biological Industries USA has partnered with Mill Creek Life Sciences, who have developed PLTMax® Human Platelet Lysate and new PLTGold® Human Platelet Lystate, which are effective alternatives to FBS and other serum supplements. PLTMax and PLTGold are produced under GMP, animal serum-free, and are generated from thoroughly screened platelets originally intended for use in hospitals.
In the poster, Biological Industries USA and Mill Creek Life Sciences examine using a combination of PLTMax Human Platelet Lysate with MSC NutriStem Basal Medium in large-scale expansion of MSCs for translational medicine.
In studying this combination for culture of MSCs, the authors focused on key areas including:
Lot-to-Lot Consistency
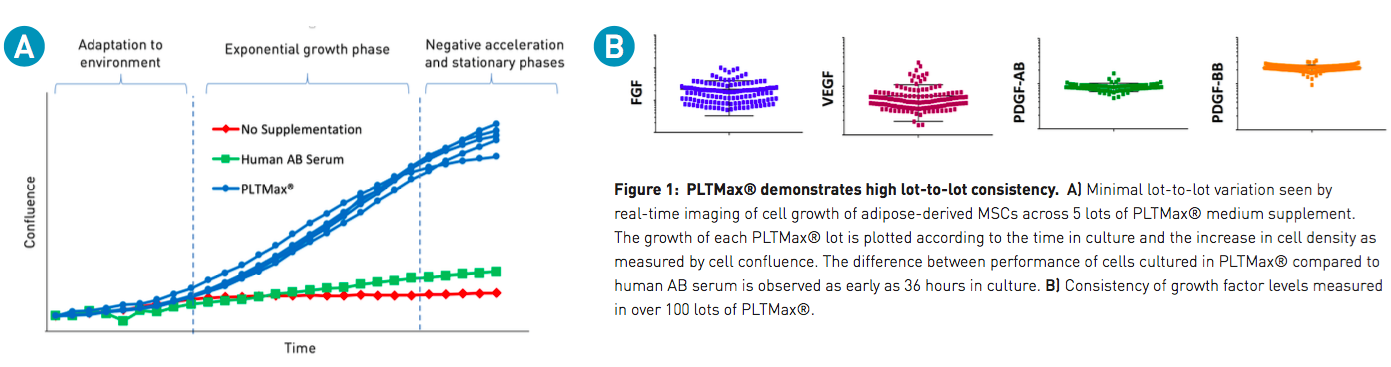
One common concern associated with animal-derived supplements is lot-to-lot variability. PLTMax and PLTGold are generated from a large pool of platelets from multiple donors, which are sourced and manufactured to produce lot-to-lot consistency. In order to demonstrate the consistency between lots, the authors examined cell kinetics (Figure 1A), and several other parameters, including consistent levels of FGF, VEGF, PDGF-AB, and PDGF-BB, measured across numerous batches of PLTMax (Figure 1B).
Performance
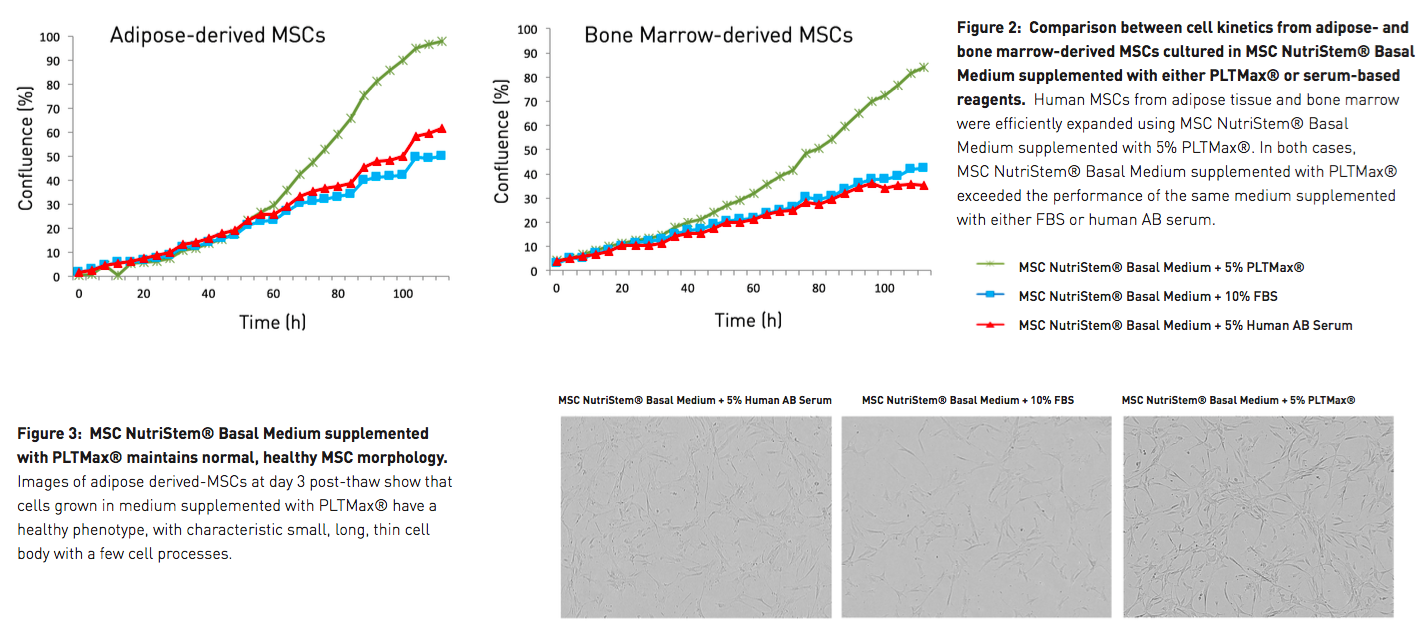
To demonstrate performance, both cell kinetics and overall cell yield were examined, and both the supplement and the basal medium were put to the test. First, PLTMax was compared against the most common serum-based MSC supplements. The authors looked at the cell kinetics of both adipose-derived and bone marrow-derived MSCs cultured in MSC NutriStem Basal Medium supplemented with either PLTMax, FBS, or human AB serum. In all cases, media composed of MSC NutriStem Basal Medium supplemented with 5% PLTMax exceeded the performance of the same basal medium supplemented with either of the serum-based reagents, with notable differences in cell confluency seen by day 3 (Figure 2). Additionally, MSCs cultured in MSC NutriStem Basal Medium and 5% PLTMax maintained a healthier morphology than those cells cultured with media containing serum-based supplements (Figure 3).
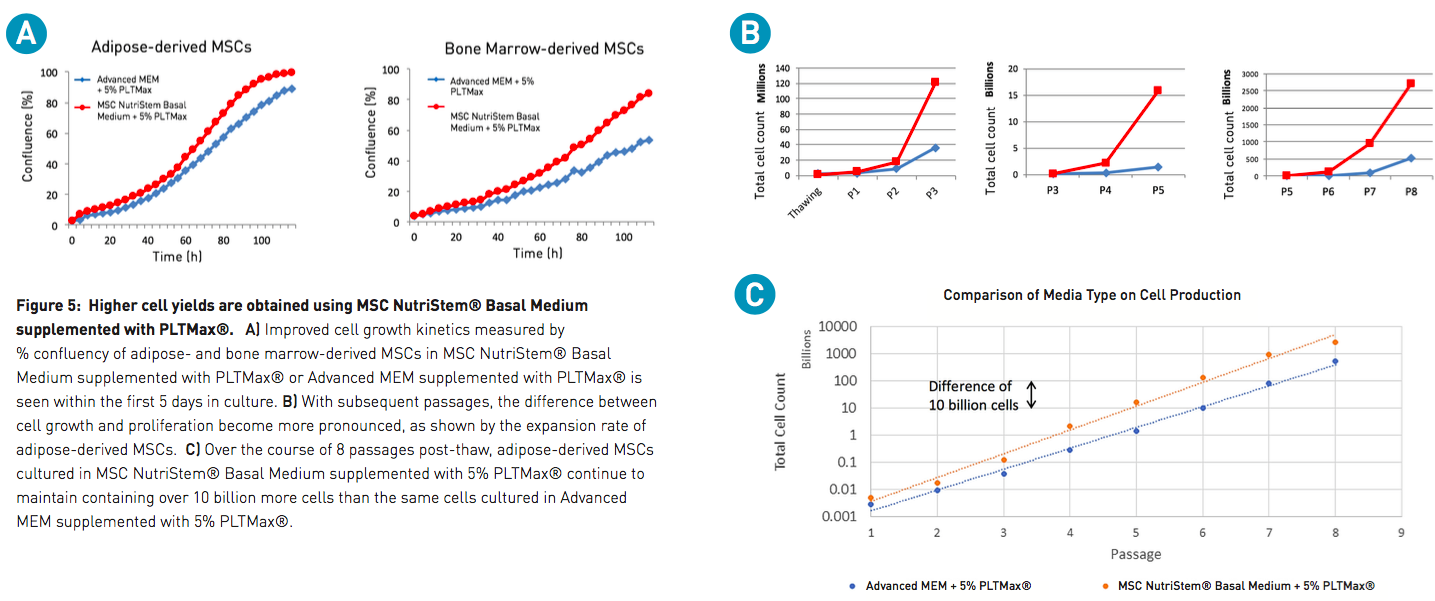
For many labs, Advanced MEM is the standard basal media for culturing MSCs. To demonstrate the improvement in cell cultures when using an optimized basal medium, cell yield in MSC NutriStem Basal Medium was compared to Advanced MEM. After the initial recovery from thaw, the growth and proliferation of MSCs cultured in MSC NutriStem Basal Medium supplemented with PLTMax was found to far outperform cultures maintained in traditional Advanced MEM supplemented with the same amount of PLTMax. Improved cell proliferation was observed within the first passage. However, subsequent passages show proliferation rates in MSC NutriStem Basal Medium and PLTMax that soon exceed Advanced MEM and PLTMax by a difference of over 10 billion cells (Figure 5), thus providing good support for the use of this combination in large-scale expansion of MSCs.
Regulatory Considerations
For Cell Therapy applications, regulatory considerations are critical. To meet the clinical and regulatory requirements, PLTMax and MSC NutriStem Basal Medium are produced under cGMP guidelines and have accepted drug master files with the FDA for accelerated use in Cell Therapy applications. MSC NutriStem and PLTMax are currently used in clinical trials around the world.
For full data and more information, please see full size of poster by clicking below.
For more information on MSC NutriStem® Medium, PLTMax®, PLTGold®, or general MSC culture, contact BI-USA at techsupport@bioindusa.com.
Other ISSCR 2017 Related Articles: