Transcriptome analysis reveals strategies for CHO cell culture media design and feed-spiking strategy to improve batch culture
When looking at batch vs. fed-batch culture, there are advantages and disadvantages to each, so considering the application is very important. Batch cultures are fairly simple, straightforward and take very little time to set up. However, batch culture typically doesn’t yield high mAb titers, due to nutrient depletion, by-product accumulation and short growth and production phases. So a batch approach may be good for lab-scale processes where product needs to be generated quickly and simply with little optimization. In contrast, with fed-batch culture, feeds are added to replenish nutrients, which increases cell concentrations, process time, and can yield much higher titers. However, fed batch culture also requires more time for optimization and resources to run. So this approach is more beneficial in situations where you would are developing long term processes or scaling up to large-scale production.
Best of Both – Batch and Fed-Batch
In an effort to find a “best of both worlds” compromise that would enable the production of higher volumetric yields in a simple batch culture, GE Healthcare and the Vienna Institute of BioTechnology looked at the addition of feed supplements only once at the beginning of a normal batch culture. These “feed-spiked” batches exhibited almost two-fold higher cell-specific antibody production and boosted volumetric productivities approximately three-fold compared with the batch control. They then applied microarrays to look at genes that could be involved with the enhanced specific productivity. Details of these studies are found in the joint presented poster, “Transcriptome analysis in high-producing CHO cell cultures: Strategies to design high-performing cell culture media”.
Study Design and Results
Culture Performance
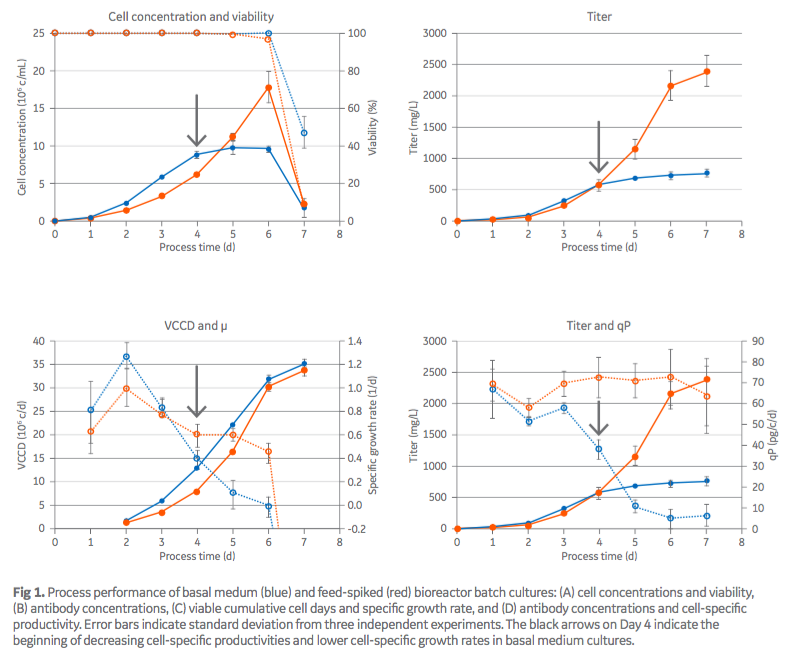
For the study, authors added concentrated HyClone™ Cell Boost™ 7a and 7b feed supplements one time at the beginning of normal batch culture to ActiPro™ basal media. Both the batch and feed-spiked processes ran for seven days. Cell viability was over 95% on both until Day 6. On Day 7, there was a drop in viability in the batch culture, which signaled the end of the run (Figure 1A). In the feed-spiked culture the cells grew slower initially, but eventually reached almost twice the high peak cell concentrations when compared with basal medium only. The authors commented that, remarkably, the integral of the viable cell concentration over the total process time (viable cumulative cell days [VCCD]) was similar between both process strategies (Figure 1C). Another difference was that in the basal medium only culture, mAb production plateaued after Day 4 (final titer 0.8g/L), while in the feed-spiked culture there was a continuous increase to three-fold higher titers (2.4 g/L) (Figure 1B). Authors stated that the higher titers could be attributed to generally higher cell-specific productivities (qP), which remained rather constant (~ 70 pg/cell/day) in feed-spiked cultures. In basal medium, the qP continuously dropped by 20% (Day 0 to 3), 50% (Day 4), and > 90% (Day 5 to 7) from 70 to 10 pg/cell/day in basal medium cultures. In average, the qP was 70% higher in feed-spiked cultures (Figure 1D).
Antibody Quality
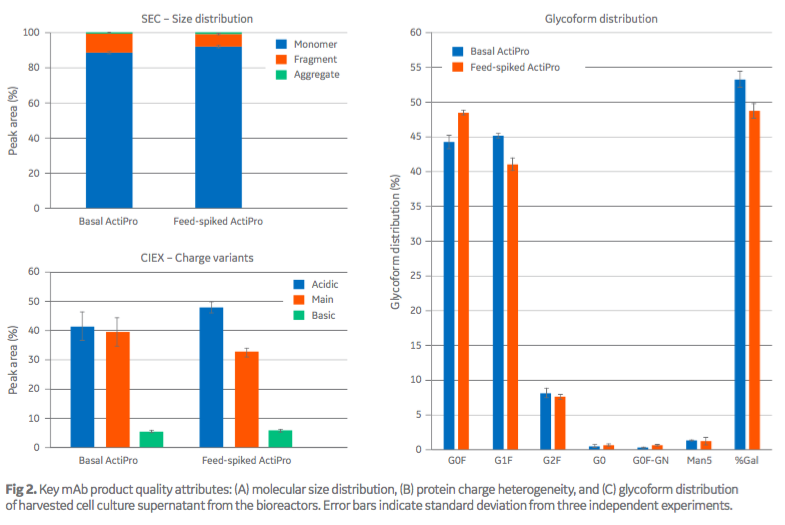
Protein quality attributes of molecular size distribution, protein charge heterogeneity and glycoform distribution were similar between the basal medium and feed-spiked cultures (Figure 2).
Statistical analysis of differential gene expression
Authors applied microarrays to look at the genes that could be associated with the increased specific productivity seen in the feed-spiked culture. Twenty-four microarrays were processed from four different days (Day 3, 4, 5, 6), with biological triplicate for each sample (3 × basal; 3 × feed-spiked) and two technical replicates for each condition. In figure 3, Venn diagrams show the intersections of up-and down-regulated genes from Day 4 through 6. In the feed-spiked medium, authors identified 332 individual genes commonly up-regulated and less than half that number of down regulated genes from Day 4 until Day 6.
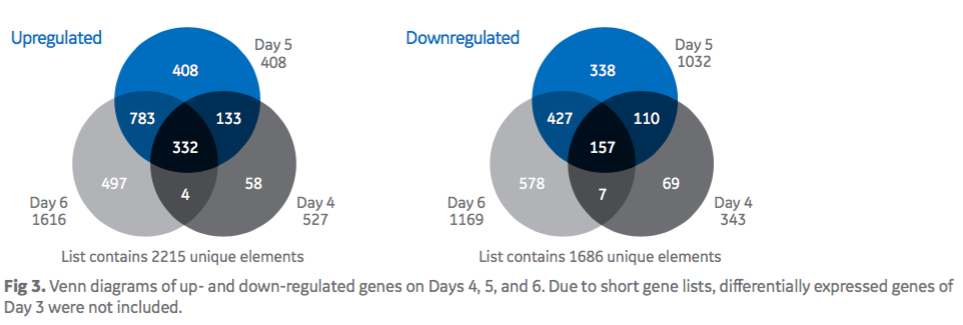
Authors stated that the number of differentially expressed genes increased steadily when the qP dropped in basal medium cultures between Days 4 to 6. This finding suggests that the genes that accounted for the observed benefit of feed-spiked cultures were continuously differentially expressed throughout the cultivation and were considered relevant to explain higher cell-specific productivities in feed-spiked cultures.
Conclusions and Future Studies
This study demonstrates that feed-spiking of basal medium can provide a simple, effective way to increase productivity in batch culture. After analysis of differential gene expression, genes that appear to be important for high cell-specific production rates were identified. This information could be useful in cell line engineering or in the design of high producing Chinese hamster ovary (CHO) media and warrants further investigation.
Additional Reading on Feed Design Strategies:
“Fed-batch culture – Optimizing feed strategies now and in the future,” The Cell Culture Dish
“Development of DoE based fed-batch strategies for high-producing CHO cell cultures,” The Cell Culture Dish
To learn more and see all the presented data, please click on the poster below to view in full size.