A Naturally Produced Extracellular Matrix that mimics stem cells’ native biochemical and structural microenvironment for improved performance
Cell and extracellular matrix interaction has been shown to play an important role in stem cell behavior and lineage differentiation, with cells’ microenvironment regulating many aspects of cell behavior.
In this mini-webinar and accompanying article, Sy Griffey, Ph.D. Chief Operating Officer, StemBioSys, presents an innovative, naturally produced extracellular matrix that provides a higher quantity of usable stem cells, that are more proliferative, and less differentiated when compared to cells grown on TCP and other comparable substrates. It is the only stem cell substrate that closely mimics the full biochemical and structural microenvironment that stem cells experience in vivo.
Dr. Griffey began the webinar by discussing the importance of cell and extracellular matrix interaction. This interaction has been demonstrated in numerous publications and has been shown to play a critical role in stem cell behavior and lineage differentiation. More specifically a cell’s contact with the microenvironment can regulate cell behavior including autocrine effects, paracrine effects, and cell differentiation.
To view the webinar, please click play below:
Artificial substrates don’t address stem cell matrix interaction
Sy goes on to explain that in the 1960’s the industry moved to polystyrene based cultureware and since that time, there has been a lack of innovation in making cell culture substrates more natural. The problem with this is that artificial substrates don’t address the stem cell matrix interaction or that cells change and respond to signals from matrices. The fact is that no surface is signal-free. Placing cells onto a foreign or biosynthetic matrix elicits a rapid response from the cells, which is not consistent with attempting to expand cell numbers without inducing differentiation.
It was this unmet need, that led StemBioSys to introduce BM-HPME® (Bone Marrow-High Performance Micro Environment). BM-HPME® is a naturally produced extracellular matrix (ECM) of proteins synthesized in vitro by bone marrow stromal cells.
BM-HPME Production
To make BM-HPME, StemBioSys uses a bank of commercially available bone marrow stromal cells from healthy young adult donors. These cells are added to the cultureware and are cultured with a proprietary cell culture media. Cells begin to divide and cover the cell culture dish or other substrate. Cells are then stimulated to create an extracellular matrix with matrix proteins deposited in a defined and ordered 3D structure. Thus creating a complex multi-protein composite that mimics the natural bone marrow niche for stem cells. After production, bone marrow cells are gently removed so as to not impact the matrix architecture or biochemistry. The matrix is thoroughly washed, solutions removed, and vessels dried leaving behind a native ECM. Analysis of the finished product confirms the complex nature of the niche with over 100 different proteins laid down by the cells. The final product is cell free with only the ECM attached to the surface of the culture vessel.
BM-HPME Performance
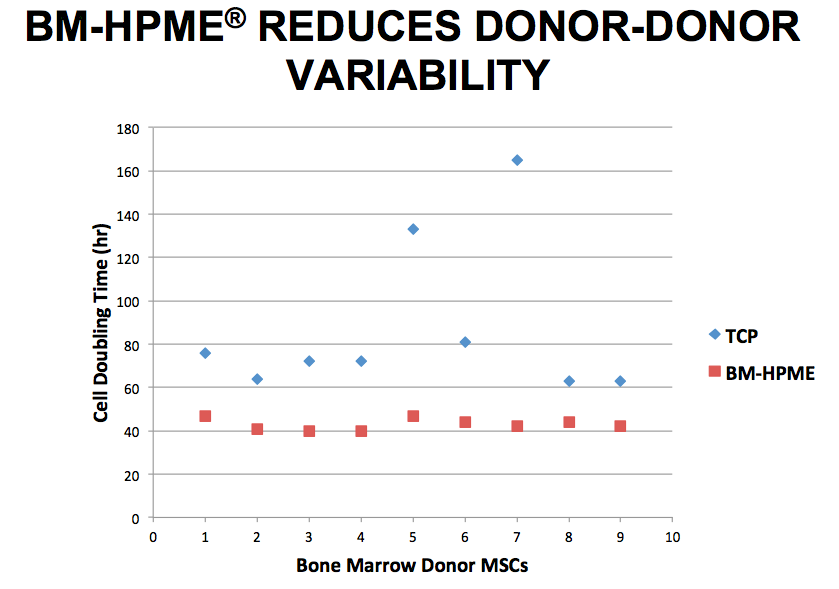
Sy stated that producing BM-HPME is not the easy approach, but it is the most natural for the cells and the presented data supports the cells’ preference for a more natural environment. For example, mesenchymal stem cells (MSCs) cultured on BM-HPME demonstrated shorter doubling times and exhibited less variation in growth potential from donor to donor when compared to standard tissue culture plates (Figure 1).
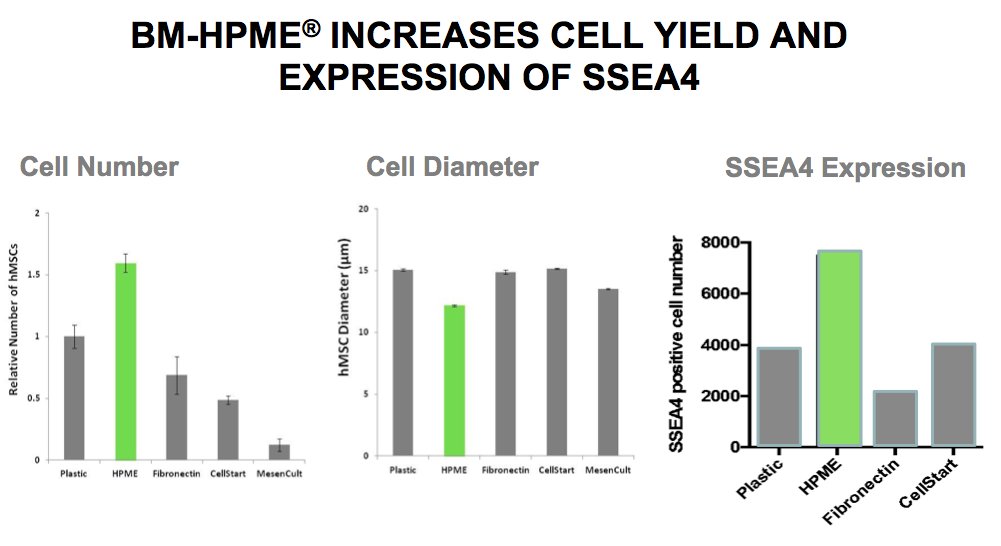
Gene map gene expression profiles showed there are marked differences between BM-HPME and other substrates. BM-HPME promotes mononuclear cell adhesion, increases cell yield, and expression of SSEA4 compared to other substrates (Figure 2).
Dr. Griffey shared that their data is not limited to MSC cultures. He went on to present data on performance of cells derived from cord blood, cord tissue and adipose tissue.
Advantages of BM-HPME
In closing Sy summarized the primary advantages of BM-HPME being ease of use, improved cell proliferation, and increased SSEA4 expression, a marker of stem cell potency. Despite the complexity of the product, Dr. Griffey said that they are able to offer pricing that is similar to other substrates.
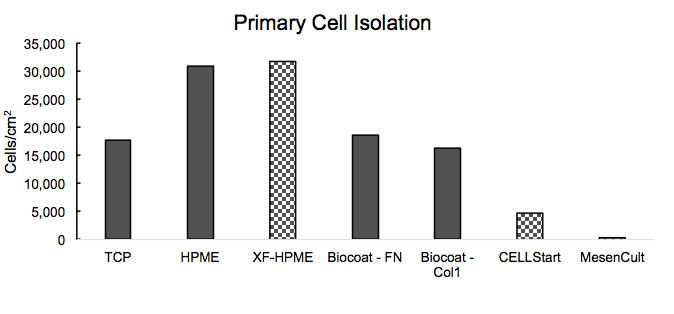
He then shared data about BM=HPME’s performance with respect to primary cell isolation and growth compared to other substrates (Figure 3)
Lastly, Dr. Griffey shared that a xeno-free version of BM-HPME is coming in 2018.
For more information please see:
“A Novel Approach for Expansion of High Quality Mesenchymal Stem Cells,” Cell Culture Dish
“Human mesenchymal stem cells (hMSCs) are a promising tool for therapeutic applications in cell-based therapy and regenerative medicine. Increasing evidence has shown that MSCs represent new hope for treating, and in some cases, perhaps even curing human diseases like diabetes, Alzheimer’s, Parkinson’s, Muscular Dystrophy , inflammatory disorders, osteoarthritis and damaged cartilage, to name a few. And although stem cells have emerged as the future of health care there are still a number of unmet needs, which must be addressed before widespread clinical and therapeutic applications become a reality. One of the major challenges in Cell Therapy is obtaining sufficient numbers of quality stem cells while maintaining their differentiation potential. BM-HPME® (Bone Marrow – High Performance Micro Environment) overcomes key obstacles to capturing and growing stem cells without losing potency…”
About the Presenter:

Sy Griffey, Ph.D. Chief Operating Officer, StemBioSys
Prior to joining StemBioSys Dr. Griffey was with Kinetic Concepts (KCI) in San Antonio for over 8 years, most recently serving as Sr Director in KCI’s Center for Advanced Research and Technology. Previous positions at KCI included Director, New Product Concept Development and Principal Scientist, Biosurgery Research.
Prior to KCI Dr Griffey spent 15 years with LifeCell Corporation serving in various roles of increasing responsibility from basic research to new product concept development to product and process development. Dr Griffey’s primary area of emphasis over 25+ years in industry R&D has been in assessing new ideas and technologies and evaluating the feasibility of turning these ideas into medical devices and products. His research and product development background includes programs and projects involving medical devices, stem cells, tissue regeneration and biologic transplant materials for orthopedic, reconstructive, sports medicine and wound healing applications. Revenues generated from products that were directed by Dr Griffey at various points in their development cycle are in the $100’sM of revenue per year.
Dr Griffey received his bachelor’s and master’s degrees at Southwest Texas State University and received his doctorate from Baylor College of Medicine in Houston with a joint appointment in Cell Biology and Biotechnology.