
Scale-Out Biomanufacturing – A Paradigm Change to Scale Up
In biomanufacturing, a production scale change is required to either meet the market growth demand or when a product moves from clinical to commercial manufacturing. How that volume is increased depends on whether a scale up or scale out philosophy is used. The industry standard has been to scale up, which translates to increasing the size of the bioreactors used in manufacturing runs. However, due to the recent availability and ease of single-use technologies, coupled with improvements in cell culture productivity; scale out may soon create a shift in the way biologics are manufactured.
Introduction
Scale out, simply put, means that bioreactors stay at smaller volumes, but the number of bioreactors used in a manufacturing run multiplies. This provides several advantages over scale up, particularly with the current direction of the industry. A few points to consider:
Changing demands
Changes to product demand require flexible and fast manufacturing scale adaptation. Increases in product demand could be caused by the addition of more indications, good post commercial results, marketing efforts, etc. Decrease in product demand can be caused by release of competitive products or the introduction of a biosimilar product. If product demand increases, bioreactor volume must be increased and/or additional bioreactor runs must be added. If demand decreases, then the problem is even larger, as manufactures may be in an overproduction scenario or significant time and resource investment will be required in scaling down. Additionally, some drug companies may be contractually bound to produce or pay for production they do not need.
Smaller Volumes
There have been conflicting reports about the future of the blockbuster drug scenario. However, with cell culture productivity improvements and more specialized drug targets, many new products are not requiring the traditional 10,000-15,000 liter bioreactor production scale in order to meet customer demand.
Multiple Products
With the heightened interest in personalized medicine as well as growth in orphan drug applications, more multi-product facilities are emerging. Not to mention the increase in outsourced manufacturing to CMOs that consistently run multiple products through their facilities. In these multi-product facilities, single use technologies enable faster turnaround time between runs with fewer cleaning and validation requirements.
A Scale Out Approach
I recently viewed a very interesting webinar on the advantages of scale out biomanufacturing. The webinar, “Evaluation of the Scale-out Biomanufacturing Strategy from Early Clinical Stage to Commercialization,” was presented by Dr. Jie Chen, Vice President of CMC Management, WuXi Biologics. The presentation covered the advantages of a scale-out strategy vs. a scale-up strategy. Key advantages include:
- Enables single-use bioreactor technology to replace traditional stainless steel, fixed-tank bioreactors in commercial manufacturing plants
- Reduces product quality and process performance scale-up risks
- Flexible process design and validation strategies
- Accommodates a wide range of product levels and market demands
Highlights
Dr. Chen began the presentation by discussing how the biomanufacturing industry landscape has evolved. It has shifted away from large scale 10,000-20,000 liter stainless steel tank bioreactors in fixed or single-product facilities for “blockbuster” drugs. Today’s “new era” of biomanufacturing facility is designed for flexibility to accommodate diverse product types and changing market demand dynamics, in addition to addressing pressures to control product costs. This is particularly true for Contract Manufacturing Organizations (CMOs), which must meet many different manufacturing demands derived from their biopharmaceutical industry clients.
Scale out is particularly well suited for a CMO. The scale out strategy provides an easy answer for facility fitness to a wide-range of products with different productivity and market demands. And a scale-out facility utilizing single-use disposable systems also provides better cross-contamination controls for multi-product manufacture while shortening the time and decreasing the cost of cleaning between different product production campaigns.
Facility of the Future
 Dr. Chen then described WuXi’s next generation biomanufacturing facility in Wuxi City, China. Currently, it is the world’s largest mammalian cell culture facility using disposable bioreactors. Created with cost control as the primary objective, this 460,000 sq.ft. facility houses 2 x 1,000 L perfusion culture capacity as well as 14 x 2,000 L fed-batch capacity. The facility can manufacture over 1,000 kilograms of product per year from either the perfusion or fed batch suites.
Dr. Chen then described WuXi’s next generation biomanufacturing facility in Wuxi City, China. Currently, it is the world’s largest mammalian cell culture facility using disposable bioreactors. Created with cost control as the primary objective, this 460,000 sq.ft. facility houses 2 x 1,000 L perfusion culture capacity as well as 14 x 2,000 L fed-batch capacity. The facility can manufacture over 1,000 kilograms of product per year from either the perfusion or fed batch suites.
Scale-out Advantages
Reduces Risk
Dr. Chen then discussed in more detail the advantages of a scale-out strategy. First, it reduces process scale-up risk. One challenge with the scale up approach is that the upstream process of “scaling up” changes the cell culture microenviroment, thus impacting product quality and process characteristics. Therefore, ensuring the success of process scale-up, from early toxicology studies to clinical and commercial manufacturing, is often a critical task. It is also a costly and resource intensive effort. In contrast, scale out eliminates scaling up because the bioreactor size stays the same throughout clinical and commercial manufacturing. Increasing scale is thus achieved by increasing the number of bioreactors.
Operating risk is also reduced. In scale up, an unexpected loss of a bioreactor would create huge financial and time losses. However with scale out, you would lose just one of the several bioreactors used in the production run, thus the material from the other bioreactors could still be harvested and product could be provided to the market on schedule.
Flexible Scale in Process Validation
Another important advantage is in process validation. As Dr. Chen points out, with a scale-up approach, process validation can only be done at the defined commercial scale. This would inhibit any adjustments to manufacturing scale based on shifts in product demand and limits flexibility over the product’s life cycle. However, in a scale-out validation strategy, process validation could occur at different scales at the same time by using a bracket validation design. Dr. Chen, then shared an example: “a process may be able to be validated at both 3×2,000 L and 6×2,000 L scale over the course of 3-4 conformance runs. Variations of this bracket design could be implemented depending on expected best and worst case demand scenarios.” Of course, validation design of each specific process needs to be discussed with relevant regulatory agent and obtain their guidance and approval.
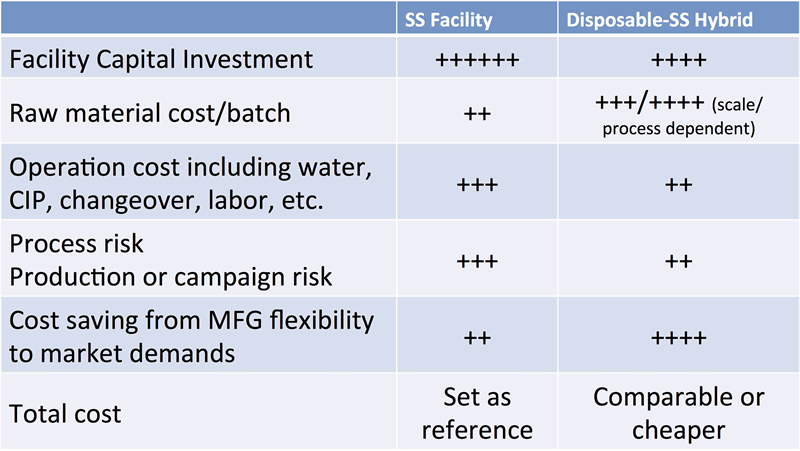
Better Cost Control
Cost control for scale out process and facility can be a challenge. Dr. Chen shared some cost reducing strategies based on WuXi Biologics’ best practice, such as utilizing continuous processing or designing a facility using a disposable/stainless steel hybrid system. Using these aforementioned factors and factoring in the initial production facility construction and validation costs, the costs per production run begin to look similar if not in favor of the scale-out strategy. Table 1, shown here, compares the overall cost of a hybrid disposable bioreactor scale-out facility vs. a commercial facility using traditional stainless steel scale up.
Cost Comparison for Stainless Steel vs. Disposable-Stainless Steel Hybrid
Regulatory Compliance
With the increasing maturity of disposables, coupled with improved understanding and guidance on extractables and leachables, concerns over regulatory compliance for single-use technologies in commercial manufacturing are quickly diminishing. In addition, regulatory agencies continue to encourage the adoption of new technologies for biomanufacturing, including continuous bioprocessing. A scale out approach allows for the implementation of several innovative biomanufacturing approaches.