3D Human Liver Spheroids: Moving Towards Meaningful In Vitro Assays
During the Society of Toxicology virtual meeting held between March 12–26, 2021, Corning® Development Associate Feng Li, Ph.D. gave an interesting presentation alongside Michael Johnson, Ph.D., CEO of Visikol, Inc., on the use and physiological relevance of 3D human liver spheroids to conduct in vitro hepatotoxicity assays and other applications. Corning offers a wide range of matrices and tissue-culture plates (i.e., Corning Elplasia® plates) as well as primary human hepatocytes to support small and large-scale production of liver spheroid cultures for drug toxicity screens.
The ability of 3D cell culture systems to more accurately recapitulate in vivo responses compared to 2D monolayer cultures across a wide variety of workflows has driven the development of technologies in the 3D culture space. In particular, one area where 3D cultures have gained popularity is in drug toxicity testing. The use of human hepatocyte spheroid cultures to assess drug-induced hepatotoxicity has been widely adopted because they are more accurately predicting liver liability, more compatible with high throughput screening and more cost effective.
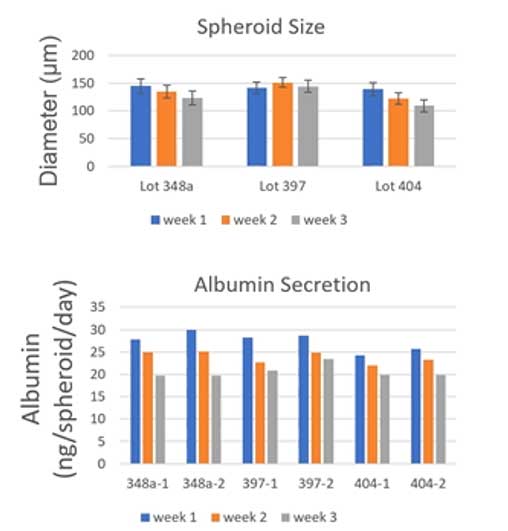
Corning’s primary human hepatocytes (PHH), pre-qualified for 3D culture, can generate liver spheroids in ~5 days and remain stable in culture with uniform morphology and stable albumin secretion over 3 weeks in a consistent manner across different lots of cells. In combination with the 24-well Corning Elplasia plates, the cells can generate 500 spheroids / well, where the total RNA isolated from each well ranged from 350 – 570 ng/mL sufficient for an array of molecular analyses (i.e., RNASeq to assess gene expression).
Dr. Li went on to announce the launch of a new product at Corning – the Corning HepGo™ Assay-ready 3D Liver Spheroid Kit (Corning Cat No. 454660) – an all-in-one liver spheroid kit for in vitro assays. The kit consists of 96 pre-plated PHH spheroids in a 96-well spheroid microplate (one uniform spheroid per well) and companion media (Corning Cat. No. 454670).
In hepatotoxicity assays, the PHH liver spheroids demonstrated 2-3-fold higher sensitivity to DILI (drug induced liver injury) compounds when compared against 2D hepatocyte cultures1.
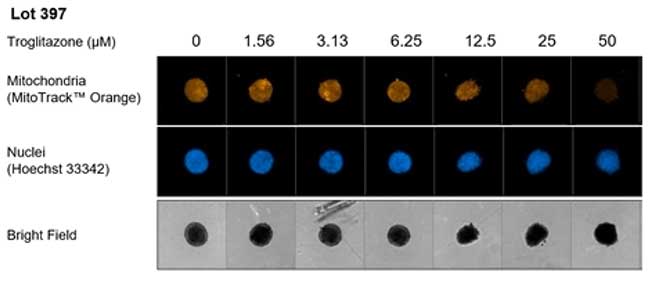
The black-walled wells of the spheroid microplates make it easy to conduct high content imaging in situ without the need to transfer the spheroids. As an example, Troglitazone-induced mitochondrial toxicity could easily and directly be visualized in the Corning HepGo kit PHH liver spheroids using MitoTrack orange fluorescent staining within the wells.
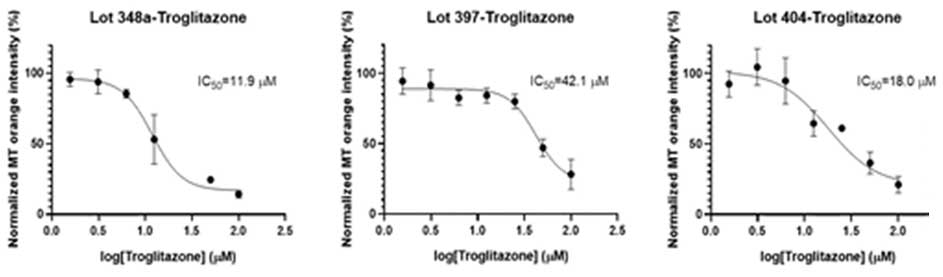
Dose-response curves for Troglitazone-induced mitochondrial toxicity enabled the calculation of IC50 across three lots of PHH liver spheroids. Donor variability was observed across the lots but this proof of principle experiment demonstrates the utility of liver spheroids to help identify mechanisms underlying drug-induced liver toxicity.
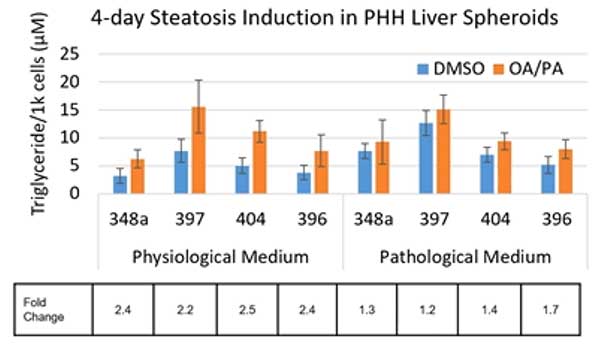
Next, in vitro steatosis induction was assessed in the PHH liver spheroids. In the experiments outlined by Dr. Li, steatosis induced by a fatty acid mixture of oleic and palmitic acids (OA/PA) was compared to DMSO controls in two 2 media formulations – one with low insulin mimicking physiological conditions and one with high insulin corresponding to pathological conditions. Lipid accumulation within the spheroids was visualized by Nile red staining and quantified using the Triglyceride-Glo assay. Across 4 different lots of spheroids, the fold increase in lipid accumulation was consistent but donor variability in the quantity of triglycerides was observed.
For the last portion of his presentation, Dr. Li covered work done at Corning to develop co-culture liver spheroids models with liver non-parenchymal cells (NPCs) and some example applications. This is relevant because there is a growing appreciation for the role of NPCs (i.e., Kupffer cells, liver sinusoidal endothelial cells (LSECs), stellate cells) in drug-induced liver toxicity, liver disease onset and progression.
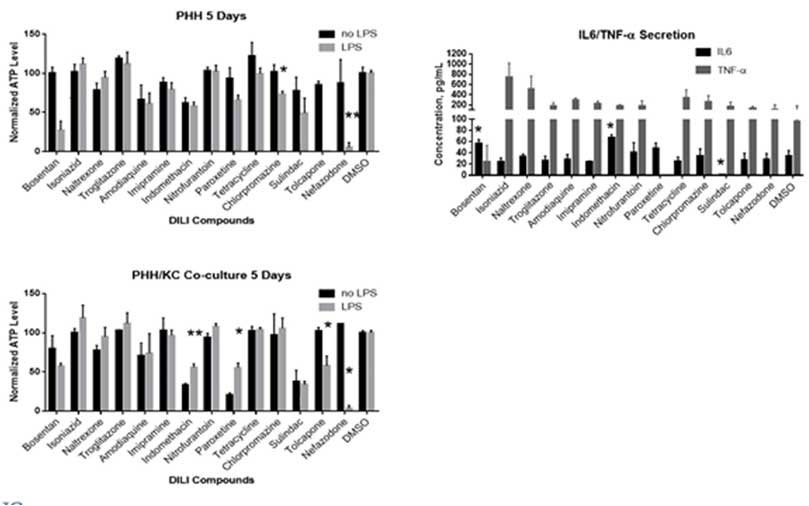
First, a two-cell type co-culture model was established by mixing 3D-qualified hepatocytes and Kupffer cells. One the co-culture model was optimized (i.e., ratio of cell types and Dexamethasone concentration), the PHH/KC spheroids were compared to PHH-only spheroids in in vitro drug-induced hepatotoxicity assays. Interestingly, KCs seems to play differential roles in hepatotoxicity when comparing PHH and PHH/KC spheroids where the presence of KCs potentiated hepatotoxicity with some compounds (Trovafloxacin) but demonstrated a protective role with others (acetaminophen), potentially related to the drug effects on IL-6 secretion levels. The observation that KCs play differential roles warrants additional testing to understand its significance.
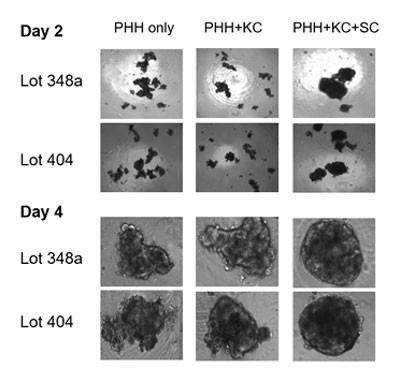
Finally, a three-cell co-culture system including stellate cells (SCs), KCs and hepatocytes was investigated to characterize lipid accumulation (steatosis induction) characteristic of liver disease. Corning researchers observed that the 3-cell co-culture system KC/SC/PHH spheroids formed 2-3 days faster compared to the KC/PHH and PHH only conditions. Additionally, KC/SC/PHH spheroids showed elevated IL-6 and pro-collagen-1a levels, contributed by the SCs and after 4-days steatosis induction, showed comparable lipid accumulation to PHH only spheroids.
About Viscol, Inc.
Visikol, Inc, is a contract research organization specializing in advanced imaging, digital pathology and 3D cell culture assays for pharmaceutical and biotechnology researchers. 3D cell culture models are more frequently and broadly used by their leading pharmaceutical clients to address a wide range of questions. Dr. Johnson notes that Visikol has incorporated the Corning HepGo Assay-ready 3D Liver Spheroid Kit for their 3D modeling assays. One of the advantages to the system is the ability to perform high content imaging directly in the culture plate. It is often challenging to image 3D models in their entirety due to their size and thickness. Utilizing the Corning 3D tissue clearing reagent enables high content confocal tissue imaging of all the cells in the model, not just the cells on the periphery to capture the spatial context of the cells within the 3D models, which provides valuable information over 2D cultures.
The Corning HepGo Assay-ready 3D Liver Spheroid Kit adds to their portfolio of 3D assays and diverse end-point capabilities to help researchers to achieve actional insights to address their research question.
Learn more at www.corning.com/lifesciences.
Footnotes
-
1. Li F, Cao L, Parikh S, Zuo R. Three-Dimensional Spheroids With Primary Human Liver Cells and Differential Roles of Kupffer Cells in Drug-Induced Liver Injury. J Pharm Sci. 2020 Jun;109(6):1912-1923. doi: 10.1016/j.xphs.2020.02.021. Epub 2020 Mar 5. PMID: 32145211.