Addressing Large-Scale Therapeutic Virus Production Using High Quality Grade PEI-based Transfection Reagents: PEIpro® product range
PEIpro®, the gold standard PEI-based transfection reagent for viral vector production
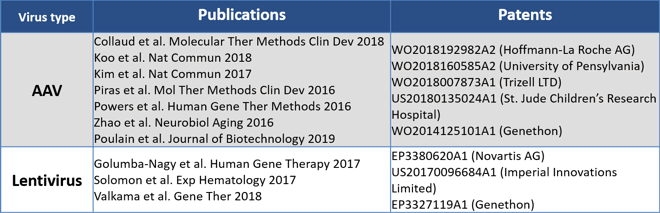
Viral vectors are the preferred delivery methods in the majority of ongoing nucleic acid therapy strategies. They are generally produced in adherent or suspension-grown mammalian producer cell lines, typically in HEK-293, HEK-293 derivatives, BHK, VERO cell lines, and increasingly into virus-specific packaging cell lines. PEIpro®-based transfection is an effective and versatile method that has been used to produced therapeutic viruses (Table 1) such as AAV and lentiviruses in both adherent (flasks, hyperflasks, fixed-bed bioreactors) and suspension cell culture systems (shaker flasks, stirred-tank bioreactors) at different production scales.
Seamless transition from process development up to clinical trials and commercialization
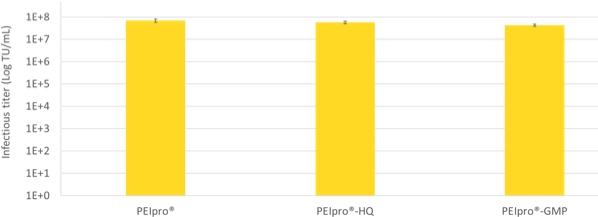
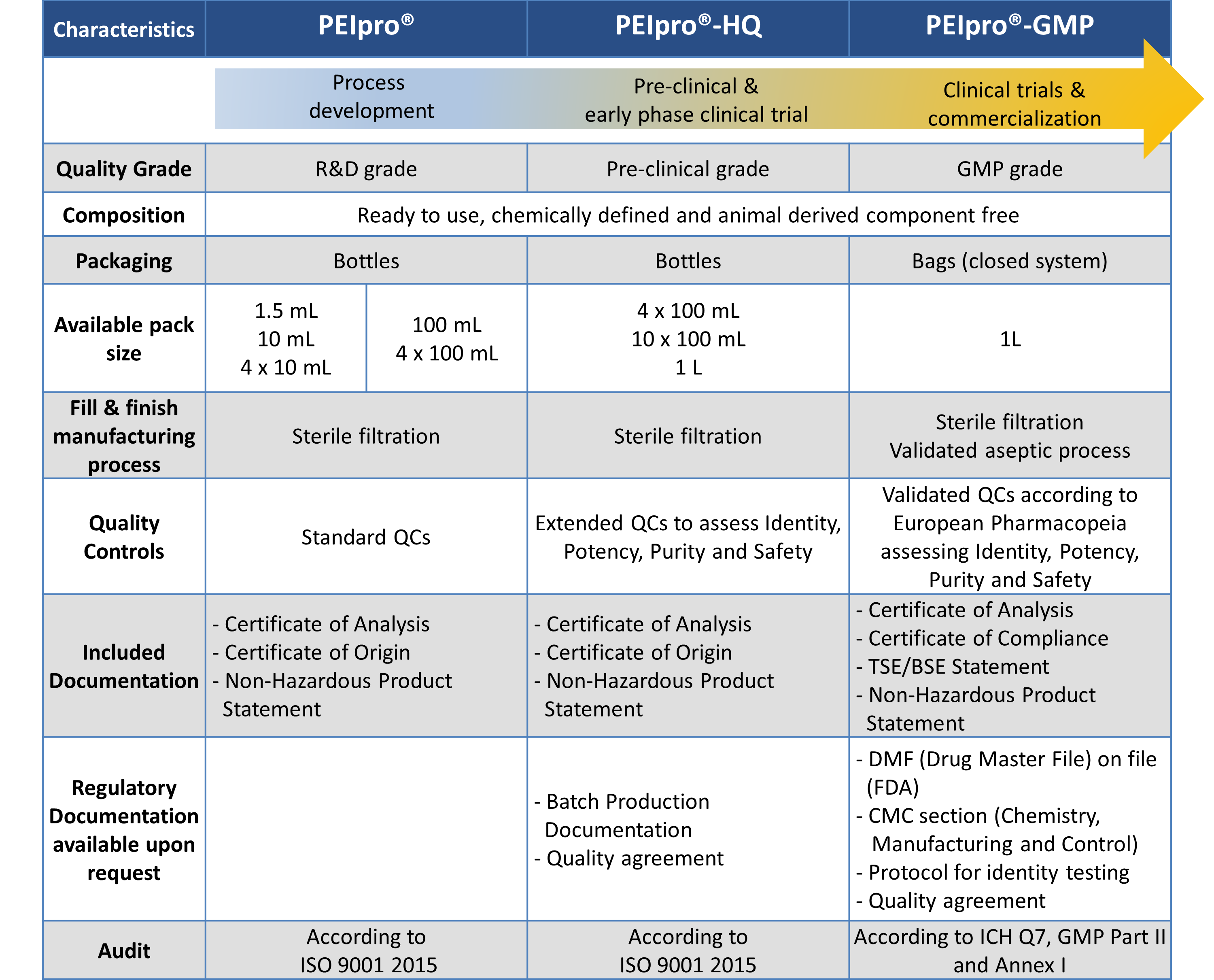
Already at the Process Development stage, it is essential to assess sourcing of raw materials to facilitate future transition to preclinical and clinical studies. At Polyplus-transfection®, we strictly apply the Quality Requirements for manufacturing of ATMPs with the availability of the identical PEIpro® transfection reagent at three quality grades: PEIpro® for initial Process Development, higher quality grade PEIpro®-HQ for pre-clinical and early stage clinical stages, and PEIpro®-GMP for late clinical stage and commercialization. Consequently, large-scale transfection protocols established with PEIpro® during process development are guaranteed to be seamlessly applicable during manufacturing of clinical batches using PEIpro®-HQ and PEIpro®-GMP (Figure 2).
Product information:
With our recently launched PEIpro®-GMP transfection reagent, Polyplus-transfection® now provides a complete PEIpro® product range to support large-scale therapeutic viral vector manufacturing from process development up to commercialization using highest quality grade PEI-based Transfection Reagents.
 Mathieu Porte, Msc, Bioproduction Project Leader provides the latest news on how Polyplus-transfection® supports the fast-growing Gene Therapy field by offering reliable and highest quality grade PEI-based transfection reagents for large scale viral vector production in adherent and suspension systems and up to clinical manufacturing and commercialization.
Mathieu Porte, Msc, Bioproduction Project Leader provides the latest news on how Polyplus-transfection® supports the fast-growing Gene Therapy field by offering reliable and highest quality grade PEI-based transfection reagents for large scale viral vector production in adherent and suspension systems and up to clinical manufacturing and commercialization.