Amplifying Adenoviral Particles in the Corning HYPERStack Cell Culture Vessel
 This article is part of a series that was published in the eBook
This article is part of a series that was published in the eBook“Cell Therapy Production”.
You can download all the articles in the series, by downloading the eBook.
Adenoviruses and other viral systems are routinely used in research and industrial applications1,2. Reports indicated that the use of adenoviruses for the delivery of transgenes is one of the most commonly used tools in both in vitro and in vivo research2. Additionally, adenovirus transductions have been recently used at both the preclinical and clinical stages in cell therapy and vaccine production leading to an increase in demand to produce more virus as efficiently as possible1,2.
To allow researchers and vaccine manufacturers the opportunity to produce even higher yields in the same spatial footprint as stacked vessels, Corning offers the HYPERStack cell culture vessel. The Corning HYPERStack vessel features Corning’s HYPER (High Yield PERformance) technology which consists of a gas permeable film as the attachment surface, eliminating the air headspace requirement in traditional vessel types. This approach provides an increase in the number of layers and corresponding cell growth surface area compared to traditional rigid single or multi-layered culture vessels.
The focus of this study was to determine the efficacy of generating amplified virus using the unique Corning HYPER technology. Standard methodologies utilize traditional stacked vessels for virus generation. Utilizing the HYPERStack vessel, researchers can generate similar titers in a smaller spatial footprint saving both time and space. The results depicted here demonstrate that the experimental approach to generate adenovirus in the HYPERStack vessel led to similar titers but larger yields compared to a standard 2-layer stacked vessel.
Methods and Materials
Cell Culture
HEK-293AD cells (Cell BioLabs Cat. No. AD-100) were maintained in DMEM without sodium pyruvate (Corning Cat. No. 10-017- CM), 10% FBS, and 1X MEM Nonessential Amino Acids (Corning Cat. No. 25-025-Cl). Transduction of HEK-293AD Cells
Cells were seeded onto a Corning CellBIND® surface 2-layer CellSTACK® (Corning Cat. No. 3310) or Corning HYPERStack 12-layer vessel, Corning CellBIND surface treated (Corning Cat. No. 10012) at 45,000 cells/cm² (0.217 mL/cm²) and incubated overnight at 37°C, 5% CO2, 98% relative humidity. The following day, the medium was removed and combined with adenovirus encoding Green Fluorescent Protein (GFP) (Multiplicity of Infection [MOI] 10). The medium was then added back to each vessel. The amount of virus (mL) added to each vessel was calculated using the following formula:
(Cells/cm²) [cm² of Well]) x (MOI 10 [IFU/Cells])
(IFU/mL)
An MOI of 10 was selected to reach the desired cytotoxic effect (<50% cells remained) in 72 hours. The crude adenovirus added to each vessel was prepared as described in Generating Crude Adenoviral Particles in the Corning HYPERFlask Vessel (CLS- AN-213). GFP expression and cell morphology were monitored throughout the course of the experiment using the Olympus IMT-2 inverted fluorescence microscope. For tips on cell visualization in a HYPERStack-12 vessel, see Corning Application Note: Options to Visualize Cells in Corning HYPERStack-12 Vessels (CLS-AN-174).
Adenovirus Harvest
The cells and medium were collected 72 hours post-transduction (Figure 1). To collect the cells from the HYPERStack vessel, PBS (without Ca2+ and Mg2+) (Corning Cat. No. 21-040-CM) was added to each vessel (0.033 mL/cm²) and incubated at 37°C for 3 to 5 minutes. To collect cells from the stacked vessel, 2 to 3 PBS washes were necessary to remove all cells. To minimize volume during freeze-thaw cycles, the cells were pelleted in a centrifuge
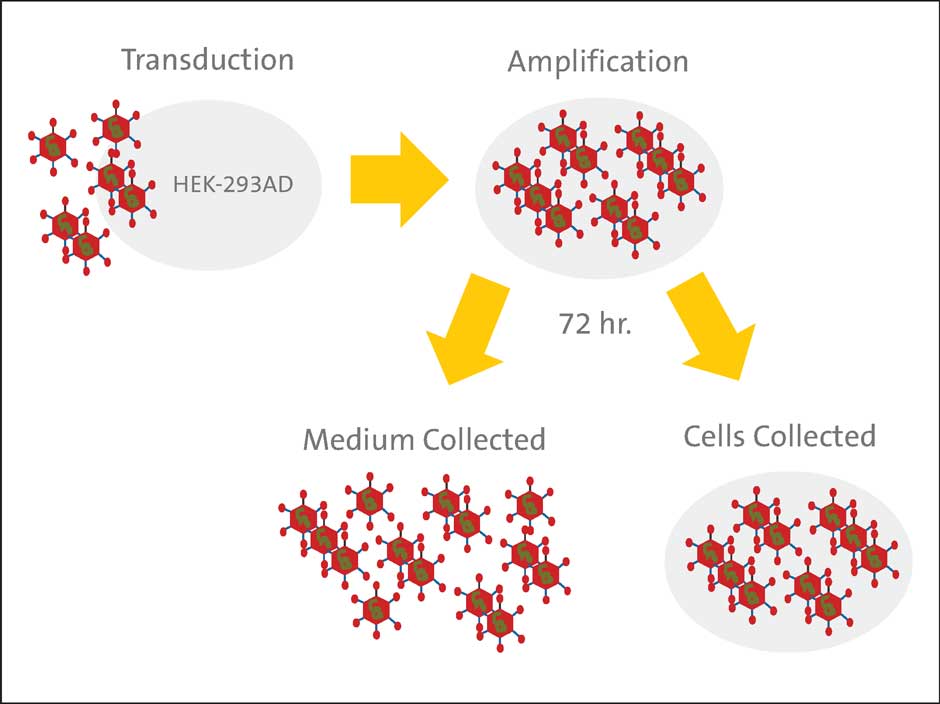
at 500 x g for 10 minutes at 4°C, and the cell pellet was resuspended in 10 mM Tris, pH 8.0, 100 mM NaCl (0.023 mL/cm²). The medium was retained, aliquoted into 50 mL centrifuge tubes (Corning Cat. No. 430921), and stored at -80°C to be titered later. The total volume of the medium was also recorded. The cell suspension was then subjected to three freeze-thaw cycles (-80°C/37°C), then centrifuged at 3,000 x g for 10 minutes at 4°C to pellet the cell debris and the supernatant containing the adenoviruses released from the cell suspension was collected. The recovered adenovirus encoding GFP was also aliquoted and stored at -80°C.
Adenovirus Titer
The QuickTiter™ Adenovirus titer ELISA kit was purchased from Cell BioLabs (Cat. No. VPK-110). The ELISA assay was performed as described previously (CLS-AN-213) and the signal in the wells was measured utilizing a PerkinElmer EnVision® Multilabel Reader.
Functional Analysis of Adenovirus
MDBK and Vero cells were transduced with adenovirus obtained from both vessels and analyzed via flow cytometry as described previously (CLS-AN-213).
Results
Cell Morphology and GFP Expression
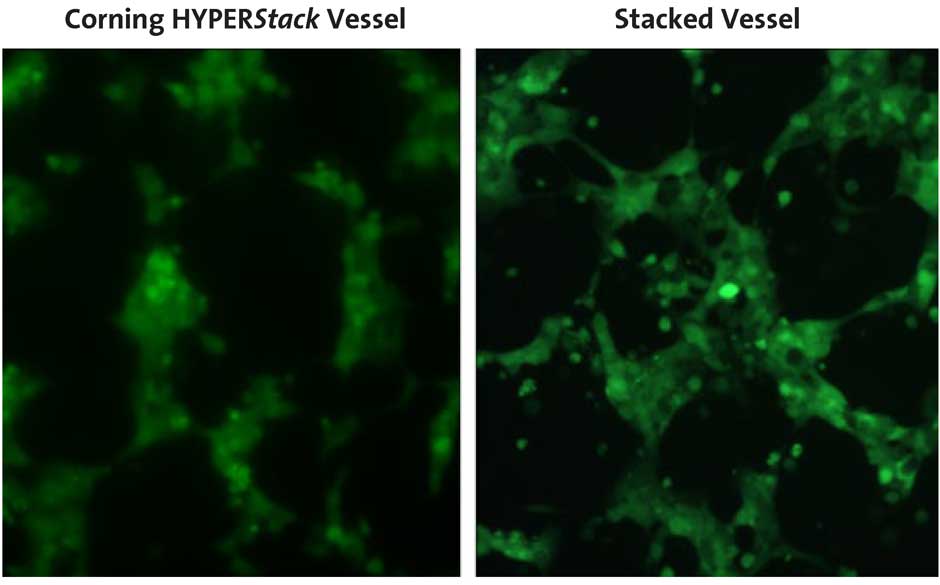
To assess adenoviral production on a 2-layer stacked vessel compared to a Corning® HYPERStack® vessel, HEK-293AD cells were transduced with adenovirus encoding GFP. GFP expression and cell morphology were monitored throughout the course of the experiment. The cells and medium were collected when less than 50% of the cell population remained attached to each vessel. Similar cell morphology and GFP expression were observed in both vessels (Figure 2) at the time of harvest.
Adenoviral Production
The viral particles obtained from either the cells or medium remained in two different fractions throughout the course of the study to (i) minimize processing and (ii) demonstrate the viral yields obtained from both fractions. For large-scale production, the cells may be lysed by either lowering the ionic strength (hypotonic shock) or with the aid of mild pressure changes that can be induced by a microfluidizer® (Microfluidics) or cross-flow filtration system³. Once collected, the titer of adenovirus encoding GFP from each vessel (either from cells or medium) was determined using the QuickTiter ELISA kit to quantitate infectious forming units (IFU)/mL. Similar titers were obtained from both vessels (Figure 3). The average titer
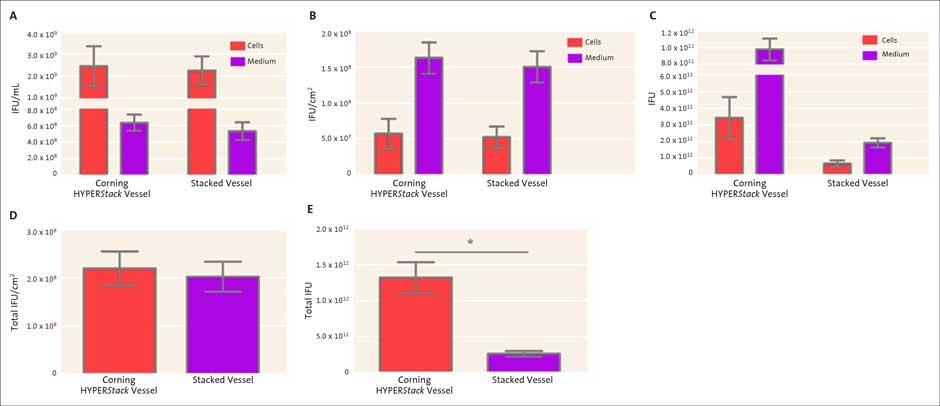
(A) Direct comparison between the HYPERStack vessel and stacked vessel titers obtained using the QuickTiter ELISA Adeno kit. (B, D) When normalized on a per cm2 basis, the HYPERStack yielded similar infectious adenoviral particles. (C, E) The HYPERStack vessel generated a significantly higher amount of total infectious adenoviral particles. (D, E) Total infectious adenoviral particles were calculated based on titers and the volume of each fraction (cells and medium) Paired t-test, * p <0.05, N=3.
obtained from the adenoviruses recovered from the cells were 2.4 x 109 IFU/mL and 2.2 x 109 IFU/mL from Corning® HYPERStack® and stacked vessels, respectively (Figure 3A). The average titer obtained from the medium was 6.3 x 108 IFU/mL and 5.3 x 108 IFU/ mL from HYPERStack and stacked vessels, respectively (Figure 3A). When normalized based on surface area, similar IFU/cm2 were observed with both vessels (Figures 3B and 3D). However, since the HYPERStack-12 vessel has a larger surface area compared to a 2-layer stacked vessel there was a significant increase in total viral yield (>5 times) (Figures 3C and 3E). These results indicate that adenovirus particles may be generated in the HYPERStack vessel with similar titers but larger yields compared to the standard 2-layer stacked vessel.
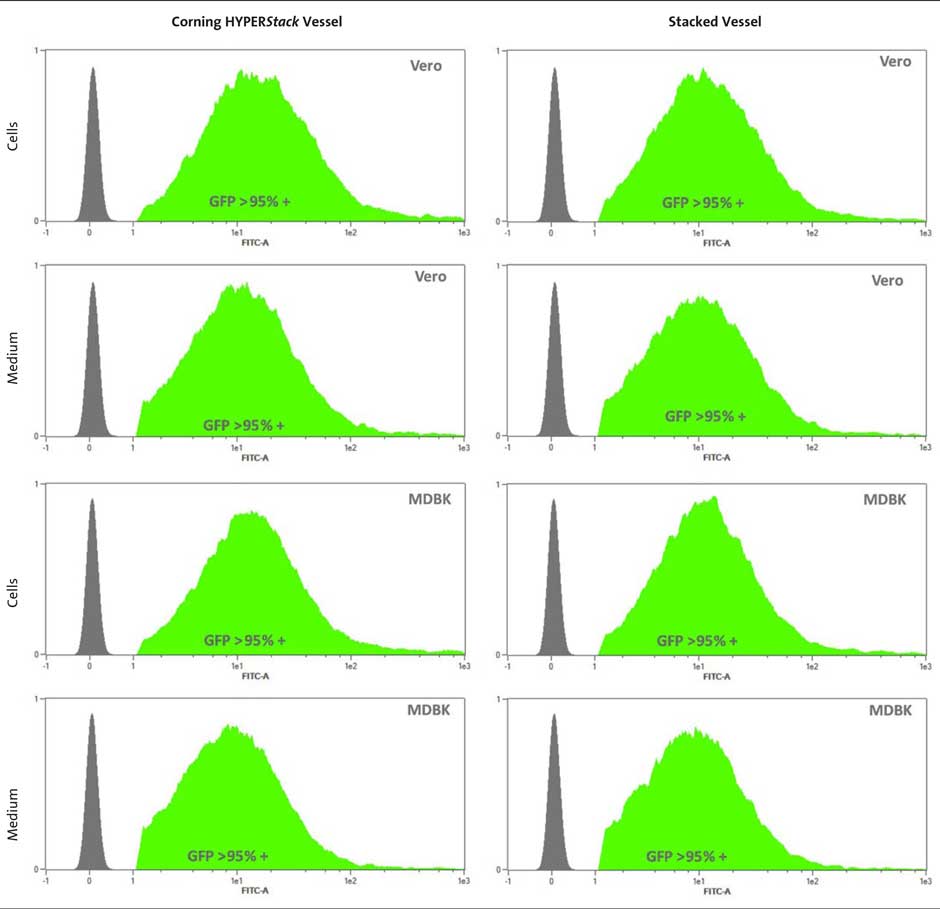
GFP Expression in Vero and MDBK Cells
To verify that the virus obtained from the HYPERStack vessel was as functional as virus obtained from the 2-layer stacked vessel, Vero and MDBK cells were transduced with amplified adenovirus encoding GFP. Each cell type was transduced with virus obtained from either the HYPERStack or stacked vessel at MOI of 100. After 72 hours, the cells were collected and analyzed via flow cytometry. After three independent experiments, the average GFP fluorescence in each cell line with each adenovirus was greater than 95% (Figure 4). Cells were transduced at MOI of 100 to ensure high expression. Previous results also demonstrated equal GFP expression regardless of the vessel when transduced at lower MOIs (10, 50, and 100) for shorter time periods (24 and 48 hours). GFP expression from each experiment varied between 30% to 95% depending on a) MOI and b) time (data not shown).
These data indicate that the virus obtained from the Corning® HYPERStack®-12 vessel is as infectious as virus obtained from a 2-layer stacked vessel.
Summary
- This study demonstrates the utility of the HYPER technology in adenovirus
- Adenoviral particles can be amplified in the Corning HYPERStack vessel at similar titers compared to traditional tissue culture vessels, allowing for greater virus production in a smaller footprint.
- Adenoviral particles generated on the HYPER technology platforms also exhibit similar levels of infectivity as in a traditional vessel.
Footnotes
-
1. Hansson, M. Nygren, P-A. and Stahl, S., Biotechnol Appl Biochem 2000. 32:95-107.
-
2. Silva, A.C., Peixoto, C., Lucas, T., Kuppers, C., Cruz, P.E., Alves, P.M., and Kochanek, S. Current Gene Therapy. 2010. 10:437-455.
-
3. Segura, M. M., Kamen, A. A., and Garnier, A. Methods in Molecular Biology. 2011. 737:89-115.