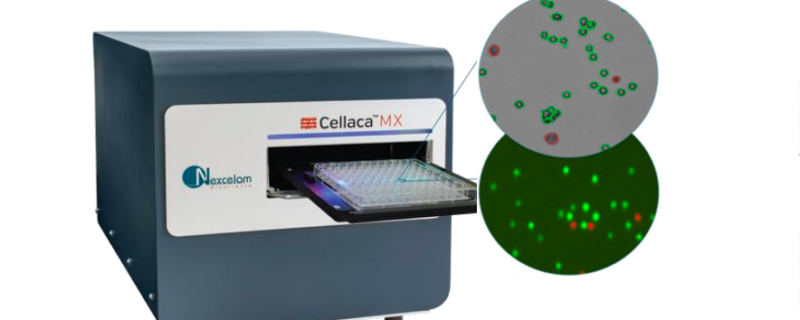
Automated, high-throughput solution for cell counting, concentration and viability
There have been many improvements in the area of cell counting and several instruments have been developed to move away from time-consuming manual cell counting. However, even with automated devices, the lack of throughput for cell concentration and viability measurements can create a process bottleneck, particularly at large-scale or industrial manufacturing. In addition, this lack of high-throughput can limit experimental design or create resource demand that isn’t feasible when large numbers of samples need to be analyzed.
In a recent poster, “CellacaTM MX: A Novel Instrument for High-throughput, High-speed Cell Counting, Concentration, and Viability,” Nexcelom Bioscience presents a solution to increase throughput and reduce process bottlenecks. In the poster, authors discuss the development of the Cellaca MX, a high-throughput automated cell counting system. The system can image, analyze, and report cell concentration and viability for 24 samples in 48 seconds using bright field trypan blue and in 2.5 minutes using multiple fluorescent imaging channels. It is compact with a small footprint suitable for a lab bench and comes automation ready with API compatible instruments.
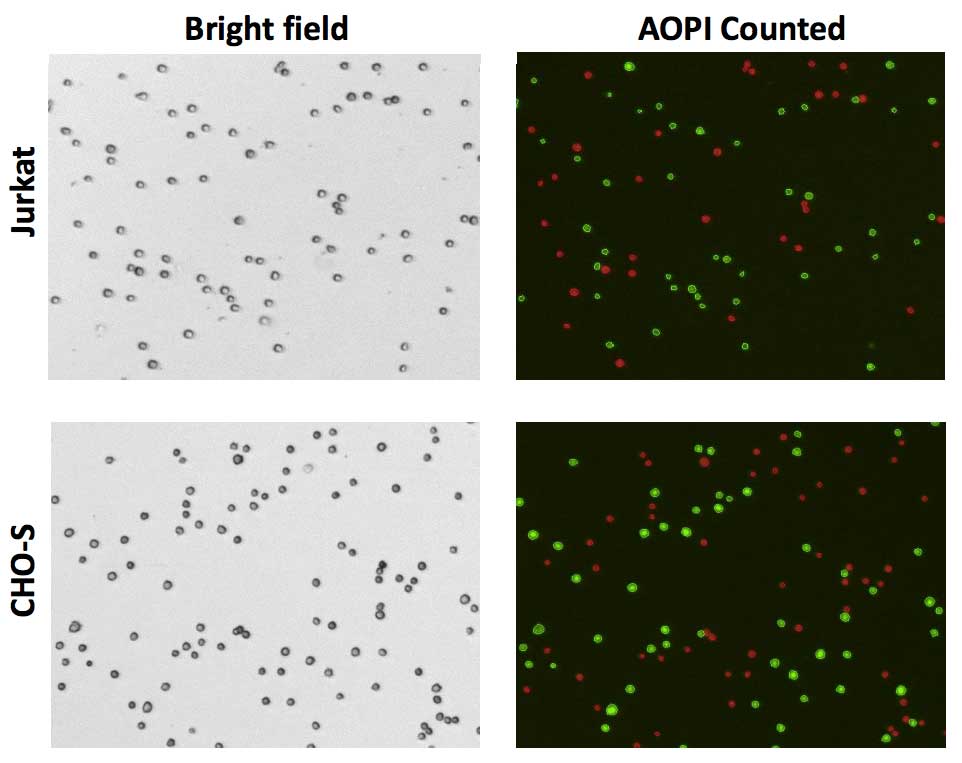
In the poster, authors present the results of their comparison between counts obtained on the Cellaca MX instrument and those obtained using the gold standard hemocytometer method. They also tested the system using CHO cells stained with trypan blue and conducted an AOPI cell viability assay on Jurkat cells.
Highlights from the poster include:
High-throughput
One of the biggest challenges to a smooth process without bottlenecks is throughput. Limited throughput can also impact experimental design as some studies will require the analysis of more samples than it is possible to run using manual or semi-automated methods. With the Cellaca MX, throughput is increased by permitting the loading and analysis of 24 samples simultaneously and the ability to use a mixing well to perform sample prep within the same plate (Figure 1). For example, 480 samples can be analyzed in 30 minutes.
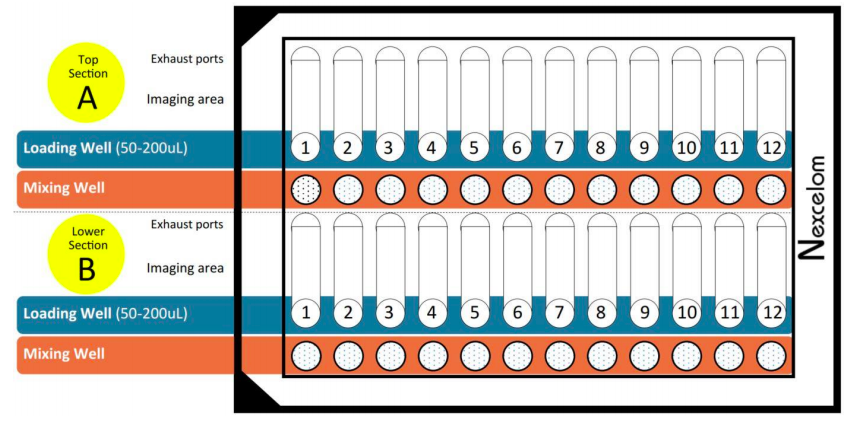
Flexibility
The Cellaca MX operates in both a manual mode and also as a fully automated, plate-based system. This permits maximum flexibility based on the project and the number of samples to be run. The system can also calculate concentrations using either bright field or fluorescence imaging. Viability can be measured using trypan blue exclusion or fluorescent viability dyes.
Low plate-to-plate variability
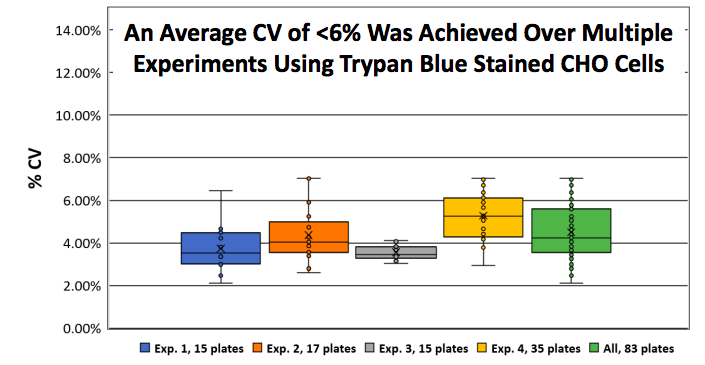
In the poster, authors demonstrate consistent counts of Trypan Blue stained CHO-S by analyzing nearly 2,000 samples with an average CV of <6%. In the study, CHO-S cells were continuously cultured over multiple weeks and the cell concentration was standardized to approximately 2×106 cells/mL. For each experiment 50 μL of Trypan Blue (0.1 % final) stained CHO-S cells were loaded into the Cellaca consumable, imaged and analyzed using the Cellaxa MX cell counter. Multiple users were able to yield an average plate-to-plate variability for Trypan Blue stained CHO-S cells of less than 6% CV (Figure 2). A total of 1,992 individually loaded samples (83 plates) were counted and analyzed in 70 minutes.
Low sample volume requirements with consistent results
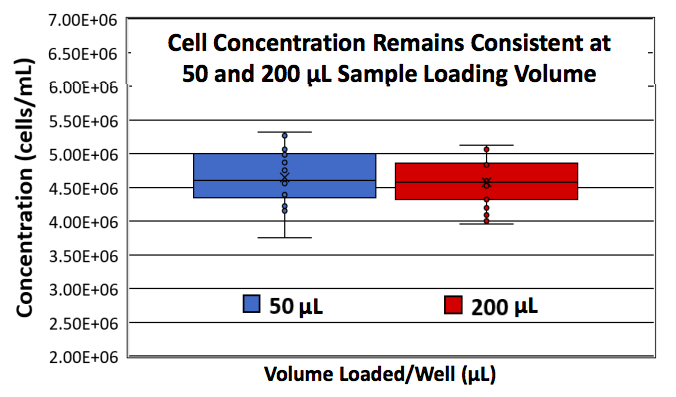
Another common challenge in sample analysis is the sample volume requirement. Frequently, there are only very small sample volumes available and a portion of the sample volume is necessary for downstream assays. With the Cellaca MX, only 25 μL of cell sample is needed. Authors demonstrated that there was almost no difference (1.5%) between loading 50 and 200 μL (Figure 3). For the study, Trypan Blue (TB) stained CHO-S cells at ~4.5×106 cells/mL were loaded at 50 or 200 μL per well into the 24-well plates, imaged, and analyzed on the Cellaca MX automated cell counter and a total of 18 plates (432 wells) per volume condition were run.
Consistent viability measurement using AOPI
To demonstrate consistent viability measurement using AOPI, three-day old cultures of Jurkat and CHO-S cells were standardized to approximately 2×106 cells/mL, then both were stained with Acridine Orange and Propidium iodide (AOPI) and analyzed on the Cellaca MX cell counter. Even at low viability (55%), the Cellaca MX produced consistent viability results. Bright field and fluorescent images were acquired for each channel and automatically saved (Figure 4). Cellular counting and analysis was performed in the fluorescent channel while the bright field image is captured for documentation.
The viability was automatically calculated and reported for each well. The average viability for 24 samples in plate 1 was 56% while the average viability for plate 2 was 53% (Figure 5).
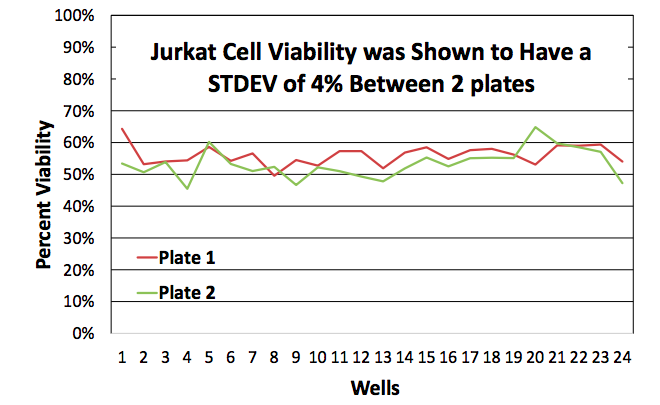
The standard measured deviation between the combined 2 plates was 4%.
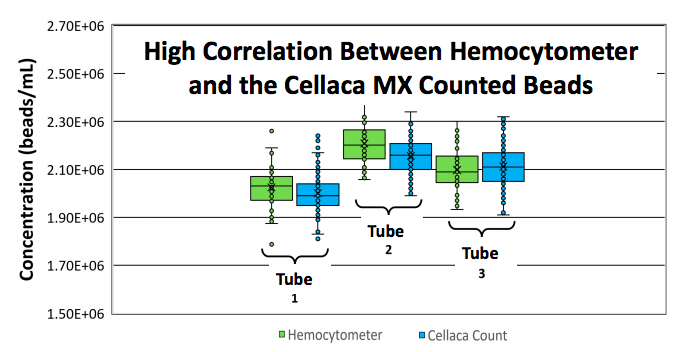
High correlation between gold standard hemocytometer and Cellaca MX
Authors performed extensive comparison studies looking at counting accuracy and consistency between the gold standard hemocytometer and Cellaca MX. Data showed that the Cellaca MX and hemocytometer counts had close correlation (1.2%, 2.3%, and 0.7% difference for multiple samples) (Figure 6).
To conduct the study, three tubes of 5 micron polystyrene beads were diluted to a concentration of ~ 2×106 beads/mL. To determine concentration within each tube, 40 individual hemocytomer counts were performed for each tube. The Cellaca MX was then used to measure the concentration on the same set of tubes.
In Summary
As the industry moves increasingly to processes that require high-throughput, instruments are needed to meet these demands. The experiments in this poster demonstrate a novel method for counting and analysis of multiple samples with high-throughput.
To learn more about the study, please see the poster in full size
To learn more about the technology, please visit Cellaca MX