
Automated integrity analysis of AAV and Adenovirus particles using MiniTEM
Guest Blog By: Vironova AB, Stockholm, Sweden
MiniTEM a compact transmission electron microscope innovation by Vironova, simplifies analytical testing of virus particles through automation. This not only saves time but also improves the robustness and reliability of the results by reducing userdependent variability. This study highlights the possibility of automated viral integrity measurements of Adeno-associated virus (AAV) and Adenovirus particles and underscores the importance of these measurements being performed in on-grid areas with appropriate stain quality. The analysis is performed using MiniTEM-enabled automated imaging, particle detection, classification and quantification. The analysis provides, a metric for determining the proportion of intact or broken particles in virus samples. In addition, as broken AAV particles may occasionally form undesired doublets or triplets, a metric for determining the proportion of such AAV particle formations is also demonstrated.
Introduction
Transmission electron microscopy (TEM) is a method used to gain information about particle morphology. A method for sample staining is typically used to provide contrast and allow structural characterization to be performed.
In Gene Therapy, viral vector stability and integrity must be carefully investigated before scaling up production. The impact of upstream- and downstream-related factors such as shear forces, pH, and temperature become increasingly critical to monitor with increasing demand for higher yields.
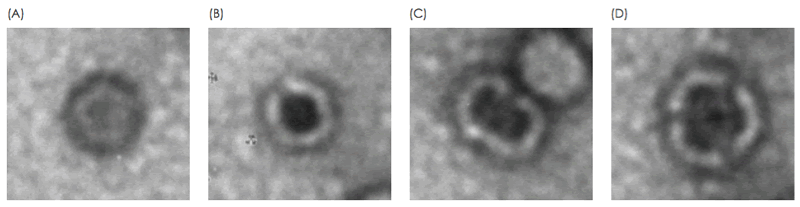
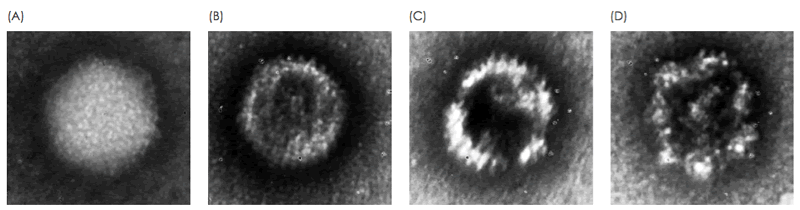
The presence of non-functional forms of the vector should be monitored, for example, when loss of viral particle integrity results from a change in process. Electron microscopy enables acquisition of detailed information on viral particle morphology. This analysis is typically performed by a trained microscopist with extensive experience in analyzing viral particles. Differences in morphologies of viral particles have empirically been found to correlate with staining patterns of the particles, where internal staining correlates with loss of integrity (Fig. 1, Fig. 2). In this study, these staining patterns are analyzed for the integrity analysis of purified AAV and Adenovirus samples using MiniTEM system. Absolute numbers from the on-grid quantitatve measurement may not correspond to exact numbers in solution, but should be considered as a relative measurement suitable for comparative studies.
MiniTEM system
MiniTEM is a 25kV transmission electron microscope system specifically designed for highly automated image acquisition and nano-particle characterization. The system combines advanced Vironova software for microscope control, automation and analysis with Delong Instrument low-voltage technologies. This combination results in a compact system that runs in any laboratory setting and that does not require specialist skills to operate. Characterization of nano-particles is performed by using a pre-configured MiniTEM script. A script is easily designed by dragging and dropping building blocks in the system software. Scripts can be configured to automatically image, detect and classify particles with different morphologies and/or size. The system thus omits the timeconsuming tasks associated with manual particle marking and measurements that often lead to inconsistent results due to subjectivity and human variability. Furthermore, since automation enables detection and analysis of a far larger number of particles than is feasible with manual analysis, the results are more consistent, statistically significant and reproducible.
Materials and methods
Sample preparation
Samples were prepared using the sitting-drop procedure and negatively stained accordingly. In brief, three microliters of sample were applied on glow-discharged, carboncoated 400 mesh copper grids and incubated for approximately 30 s. Excess sample was blotted off and the grid was washed with water and stained using 2% uranyl acetate for 10 s. Finally, the stain was blotted off and the grid was left at room temperature to air dry.
Automated image acquisition and analysis
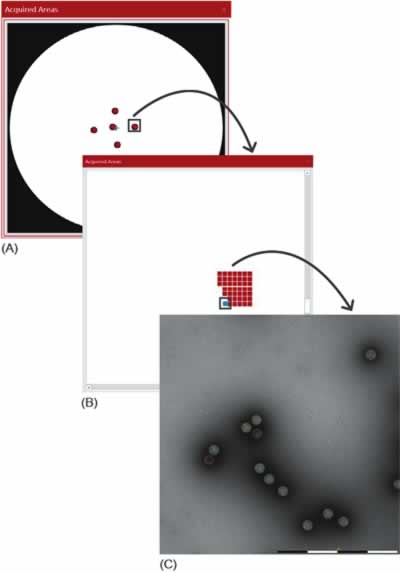
Large areas at multiple locations on the grid are analyzed efficiently thanks to the automation built into MiniTEM system. MiniTEM software has building blocks that through dragand- drop functionality can be configured to design a script for different types of analyses. In this study, a script from the MiniTEM analysis library was designed, which automatically acquires and analyses images at five locations (a cross-pattern) on the grid, a set of images is acquired at each position, each with a field of view (FOV) of 1 and 2 μm for the AAV and Adenovirus samples, respectively. Particles are then detected and classified based on area and staining pattern (Fig. 3). A total of 11,101 Adenovirus particles and 44,901 AAV particles were detected and classified in this study.
Results
Integrity analysis of AAV particles
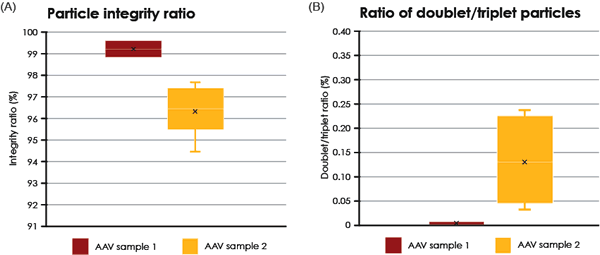
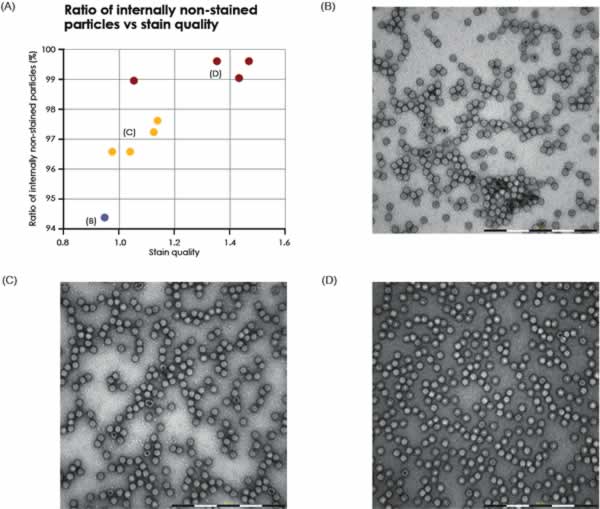
Two different samples of purified AAV particles were automatically imaged, analyzed and compared. In TEM, intact AAV particles are defined as those who exhibit a lack of internal staining because of a maintained structural integirty or the virus capsid. In contrast, AAV particles with internal staining are presumed to be non-intact. For each sample, the proportion of internally unstained AAV particles (intact) as well as the proportion of AAV doublets and triplets was analyzed (Fig. 4). The formation and biological relevance of the doublet and triplet conformations are not known but could be linked to a disassembly and self re-assembly process occurring under sub-optimal conditions. It was shown that AAV sample 1 has a larger portion of intact particles compared with sample 2 (99% intact particles compared to 97% in sample 2 Fig. 4A). In addition to the single AAV particles detected, 42 doublets or triplets (0.125%) were detected in sample 2, whereas in sample 1 no doublets or triplets were detected.
The internally stained AAV particles can possibly be linked to a disturbed protein assembly or lack of interior support such as genomic material.
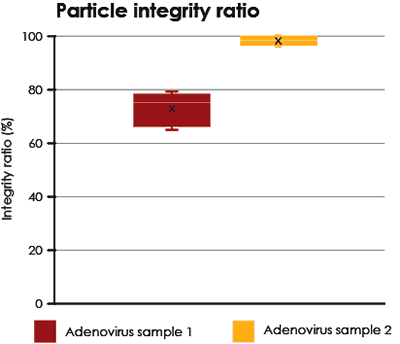
Integrity analysis of Adenovirus
Two different samples of purified Adenovirus were analyzed and compared. The proportions of intact particles are shown in Fig. 5.
Accuracy of the analysis
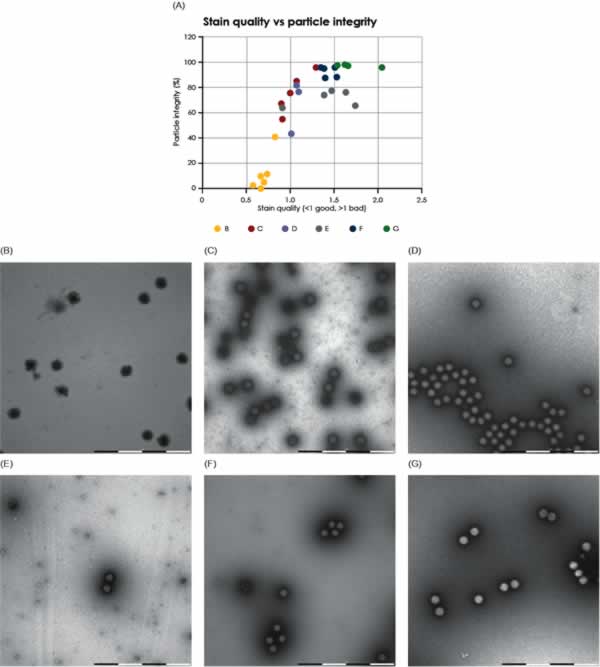
This study used the staining pattern as a marker to identify intact or broken viral particles. Intact particles were defined as those particles that did not show internal staining; consequently, internally stained particles were defined as broken. This marker has previously been empirically validated in manual studies performed by trained microscopists using conventional TEM. However, when assessing particle integrity, it is critical to avoid areas where poor stain quality biases the results. To avoid these areas, the MiniTEM script was designed to also evaluate the stain quality. The correlation between stain quality and loss of particle integrity was investigated and is graphically shown in Figs. 6 and 7 for AAV and Adenovirus samples, respectively. The results show a significant correlation between staining quality and particle integrity in areas with poor stain quality, whereas integrity is stable in areas with good stain quality. Algorithms were developed and applied when designing the MiniTEM script to automatically exclude areas with poor staining and are thus enabling accurate on-grid particle integrity studies to be performed automatically.
Conclusions
This study demonstrates that viral particle integrity can be accurately measured in on-grid sampling areas where the stain quality is appropriate. Since the MiniTEM script can be designed to automatically analyze viral particle integrity in well-stained areas by excluding poorly-stained samples from the analysis. MiniTEM is able to quickly image, detect and classify particles with a high degree of accuracy. This enables the MiniTEM system to present a reliable and reproducible metric of the proportion of intact or broken particles on the grid.
Process operators can therefore use MiniTEM to objectively and reliably compare the stability of viral particles at different formulations or steps in the purification processes. Since the analysis is quick and easy to perform, viral integrity data are typically obtained in about one hour.
For more information, please visit www.vironova.com
Related Articles:
- Direct vs. indirect methods for characterization and analysis of subvisible particles – A comparative study
- Automated Adenovirus Purity Analysis Speeds Viral-based Gene Therapy Process Development
- See the Quality of your Viral Particles at Different Steps of your Process