Automating Osmolality Monitoring in Upstream and Downstream Bioprocessing
Osmolality, the concentration of a solute in a given solvent, is a frequently monitored critical quality attribute (CQA) in bioprocessing. The need to test osmolality is present throughout upstream and downstream bioprocessing. However, many current methods for monitoring osmolality are inefficient and not geared to support high throughput. Developers are increasingly looking to make critical product decisions earlier in process development. This requires testing more conditions in a shorter timeline and making the results quickly and easily accessible. For companies to have the confidence needed to make these decisions, the data generated in testing must be accurate and have low variability. The increase in testing and data generation has resulted in bioprocessing incorporating more high-throughput and automation solutions. Thus far there has not been an osmolality monitoring solution that can support this level of high throughput with accurate, precise results.
Why is Osmolality Important?
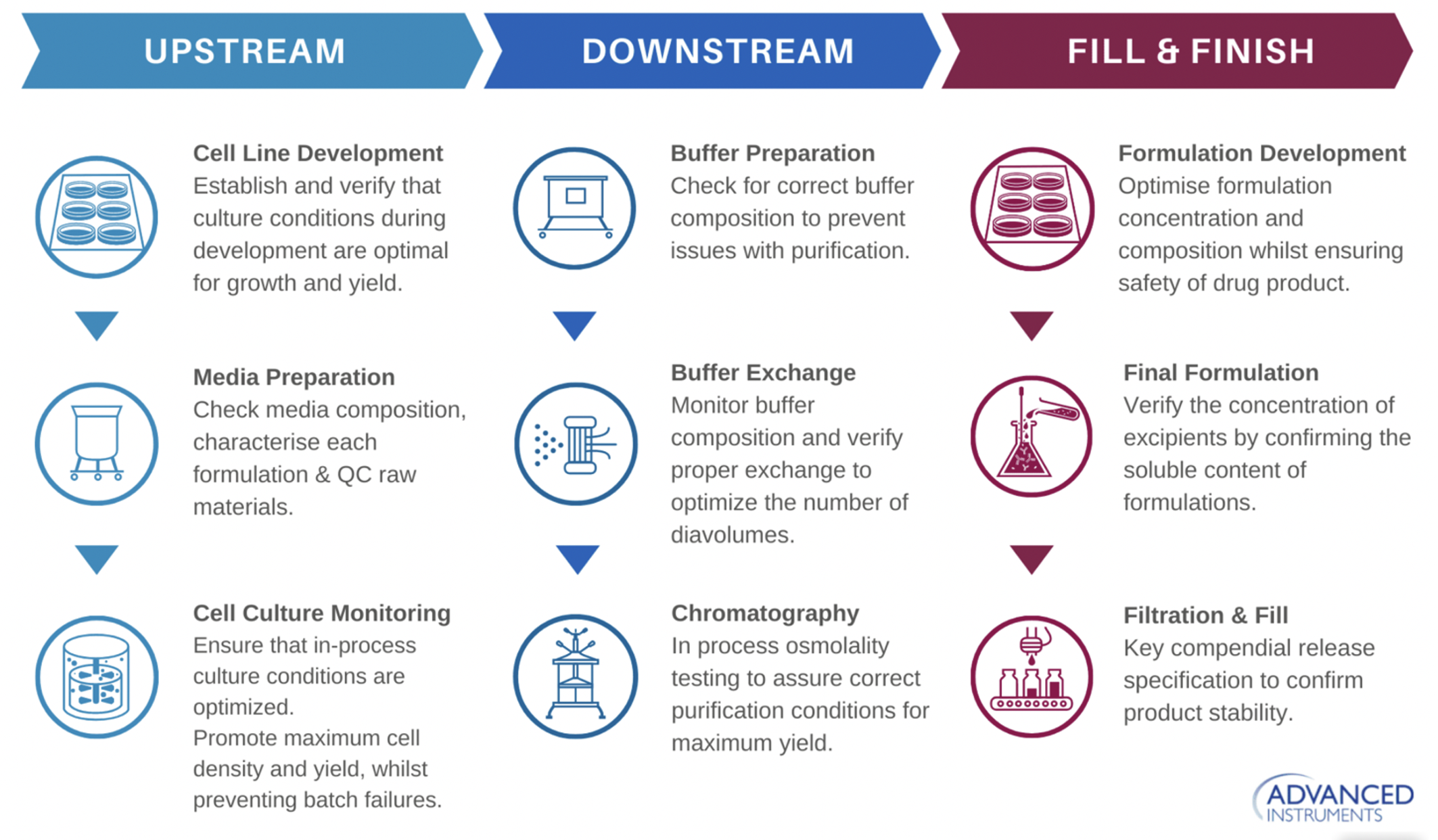
Osmolality testing is an analytical measurement important in all stages of bioprocessing including upstream and downstream process development and formulation development. In upstream process development, osmolality testing is used in cell line development and media development/optimization. It is also used for cell culture monitoring during process development and manufacturing. In downstream bioprocessing, osmolality is used for buffer composition/exchange and for finding ideal purification conditions. Process development requires testing many conditions to identify the ideal process and ensure the highest productivity and product quality. Therefore, many data points are required, which necessitates a high throughput process that can handle high volume sample testing.
Osmolality is also used extensively during formulation development to identify the ideal formulation and to find appropriate conditions for stability. This requires a high volume of samples to be run to test different formulation concentrations and compositions to ensure safety. Additionally, high volume screening is needed to optimize stability by testing a variety of conditions at several time points. In final formulation, osmolality is used to verify the concentration of excipients by confirming the soluble content of formulations.
For GMP (Good Manufacturing Practice) manufacturing, osmolality testing is used as a process parameter as well as a critical quality attribute for final product QC (Quality Control) and release (Figure 1).
Osmolality Monitoring
Osmolality monitoring and testing is still mostly conducted manually. Often it is conducted using a single sample model, introduced one sample at a time, with a run time of 90 seconds to two minutes to receive a result. This method is a time consuming and manual process. Another method incorporates some automation by using an instrument with a turntable format that allows the loading of up to around 20 or 48 samples at a time. In this scenario, the operator still manually pipettes the sample into tubes and physically loads it onto the device. Manual pipettes also require a larger sample volume at over 100 µL This process must be repeated until all samples are tested.
These manual operations may be sufficient if only one or two tests are needed. However, when the situation calls for many samples, for instance in cell culture monitoring and media optimization or formulation, it is common to have over a hundred samples in the queue waiting for testing. These more manual operations often can cost an operator one half to one full day running samples. In addition to being time consuming, manual operations can also introduce variability through operator error and handling inconsistencies. This creates errors resulting in inaccurate data and high variability. Lastly, these methods require higher sample volumes, which are at a premium during process optimization when sample material is in short supply.
These challenges created a need in the industry for a better solution that reduces the manual aspect of osmolality testing and operator time, decreases opportunity for errors and variability, improves throughput, and lowers sample volume requirements.
The OsmoTECH® HT Automated Micro-Osmometer Solution
To solve these challenges, Advanced Instruments launched the first 96 well plate-based osmometer, called the OsmoTECH HT Automated Micro-Osmometer. It was designed to support automated high throughput testing and significantly reduce the time, cost, and opportunity for error associated with existing osmolality testing methods. In addition, it requires just a small sample volume, as low as 40µL for early process osmolality testing and 20 µL for STAT testing.
Automated Testing Solution
Currently most osmolality testing is done offline or at-line. In the manufacturing space, where samples are usually tested one at a time, this may be sufficient. However, when doing process development or formulation work, the large number of samples that need to be tested make this method unmanageable. Companies have tried to solve this by using separate instruments offline that permit multiple samples, but none offer the compatibility or efficiency of a 96 well plate.
With the OsmoTECH HT, samples are loaded into SBS-compliant 96 well plates
with a unique barcode for full traceability. This format is also compatible with other sample testing platforms commonly used in bioprocess workflows for testing critical process or analytical parameters. These process development workflows are often automated using a robotic system, like the Tecan®, that takes the primary sample and pipettes it into plates. This allows operators to place the 96 well plate on the robot where the primary aliquot will be pipetted and 40µL will be placed into each well. Then they can take the plate and place it on the OsmoTECH HT for testing, then the next plate can be placed in and so on. Operators can load up to two plates onto the device at a time for a total of 192 tests. Furthermore, up to 1,000 tests can be performed before consumables need replacement. The automation enabling OsmoTECH HT provides seamless integration with Ambr®, Cedex®, and liquid handling automation systems. This permits sample testing without the need for operators to be in the lab overnight, on weekends, or on holidays. Existing methods require extensive “hands on” operator time. Since the OsmoTech HT allows automation of much of the work, it frees operator time to work on critical tasks like data analysis.
The OsmoTECH HT is the only osmometer that uses freezing point depression, the gold standard method and technology for measuring osmolality, and has the throughput and automation required for large scale testing.
Data Integration
Flexibility is key to integration as different companies are at various stages of automation and digitization. The OsmoTECH HT is flexible to fit into any kind of workflow. It can support a local printer and be integrated directly within a digital ecosystem that has all instruments connected through software.
In a fully digital organization, anyone with permission can review the data set from wherever they are, thereby maximizing the time that teams are spending in the lab and enabling employees to work from multiple locations. Supervisors can set up customizable templates for operators with sample IDs for the tests they are going to run. These templates reduce set-up time when testing large numbers of samples in a high-volume workflow. Essentially, operators place the plate in, hit start, and walk away.
Data management is flexible with the option to save data to a network database, a USB device, or web server. Sample identification and instrument control can be automated using bi-directional communication to save time and eliminate transcription errors. Then results can be integrated into a data management system and batch records using OPC-UA. In addition, data integrity features ensure 21 CFR part 11 compliance and use in a GMP environment.
Accurate and Reliable Results
In a recent application note, Advanced Instruments OsmoTECH® HT Automated Micro-Osmometer: Delivering Reliable Performance of Osmolality Testing with High Throughput Automation, data was presented to demonstrate the OsmoTECH HT’s high accuracy and precision with no-carry over or edge effect.
Sample carry-over
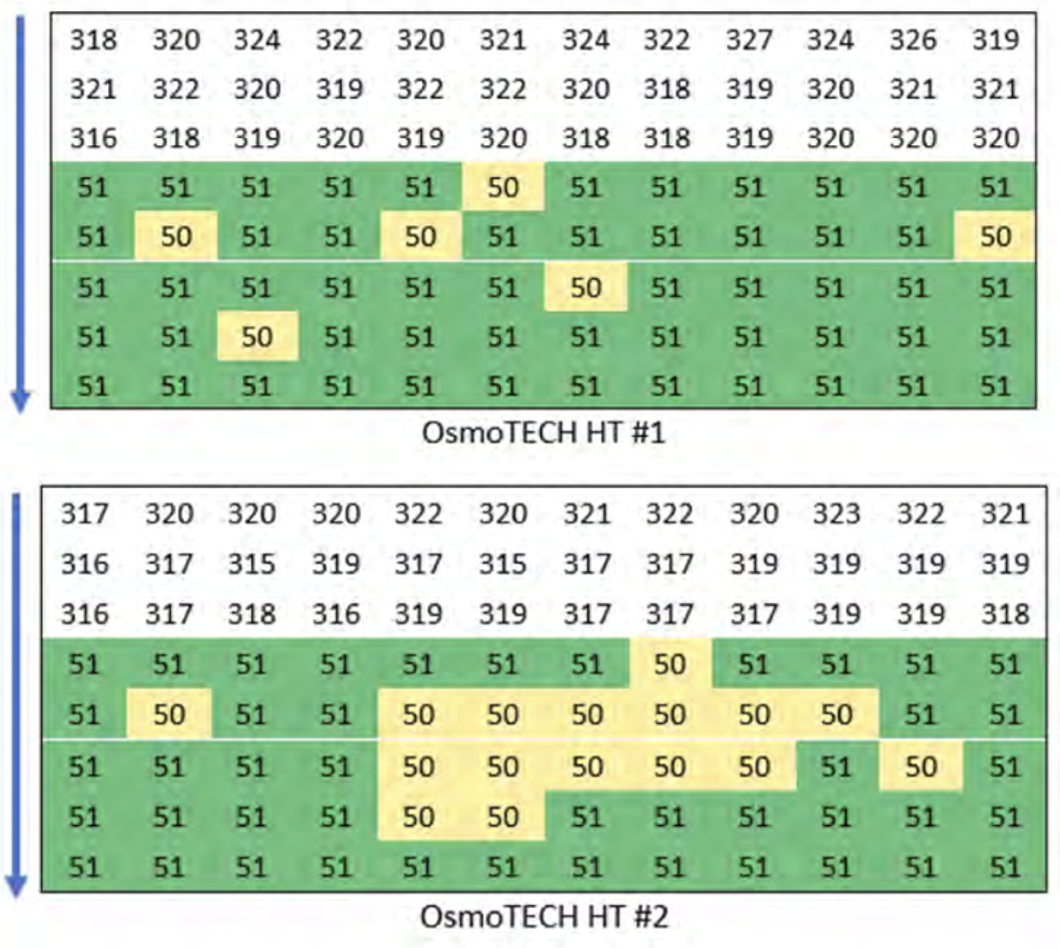
In the study, carry-over analysis was conducted to demonstrate that the sample probe used in the instrument did not cross-contaminate subsequent wells. Thorough cleaning of the pipette and sample probe is automatically conducted between each sample.
testing was performed on two OsmoTECH HT instruments using ProtinolTM 320 as the high-level sample and the 50 mOsm/kg salt standard as the low-level sample. Testing on the 96 well plate was column-based and began at the top of the plate as indicated by the arrow in Figure 1’s heatmap. Carry-over indicated by the low-level controls showing results higher than the expected value. Therefore, no evidence of carry-over was detected from the high-level sample into the low-level sample.
User-friendly
The OsmoTECH HT Automated Micro-Osmometer features an intuitive operating system and easy to use touchscreen interface. The touchscreen has a step-by-step guide to allow users to find the status of tests and track remaining consumables. It is also backed by a knowledgeable support team that is readily available for training and support.
First-in-Class OsmoTECH HT Enables High Throughput Osmolality Testing
This built for automation system facilitates process development and formulation development with high throughput capability. It easily fits into existing workflows for monitoring critical quality attributes using just a small amount of sample for each test while delivering accurate and precise testing results. Since the instrument connects directly into the data ecosystem, testing results are easily and rapidly accessed. All this is achieved via an intuitive operating system and touchscreen interface that offers a step-by-step guide allowing users to access the status of tests and consumables. Lastly, Advanced Instruments offers a knowledgeable support team for training and support.
To learn more, please see OsmoTECH® HT Automated Micro-Osmometer
For further reading:
Osmolality Testing in Advanced Therapies
In addition to the more traditional protein-based biologics that are focused on in this article, osmolality testing is an important part of bioprocessing in emerging modalities.
Cell and Gene Therapy
There has been increased interest in looking at osmolality testing for cell and gene therapy production. The role of osmolality testing and cell and gene therapy was highlighted in a recent white paper, “8 Ways That Osmolality Testing Improves Cell and Gene Therapy Process Development and Manufacturing”. In the paper, eight key areas are discussed with respect to their connection with osmolality.
One area discussed is the effect osmolality has on transfection. Studies have shown that maintaining osmolality is important when transfecting cells with plasmid to initiate viral production. It is also theorized that osmotic pressure plays a role in the stability of the internalized PEI:DNA complex, which is particularly important for HEK293 cells that commonly undergo polyethyleneimine (PEI)-mediated transfections. It has been hypothesized that the increase in osmotic pressure caused by entry of the complex protons into the trafficking endosomes allows for the release and delivery of the DNA to the target cells. This, along with other variables, must be considered and characterized early in process development to establish reliable and reproducible methods.
Osmolality also influences the ability of buffer solutions to cross cell membranes, so it is imperative to set specifications around the osmolality of media and transfection cocktails. For example, during cell lysis to release cell-associated adeno-associated virus (AAV), osmotic shock is one method used. This method relies heavily on the osmotic pressure of the cellular environment. Incubation at high salt concentrations (high osmolality) can expose the viral genomes but it could also potentially damage them. Establishing a tight specification range for the osmolality is essential to develop a controlled and repeatable process for optimal titer and yield.
Osmolality plays a key role in vector production and stability. Vector stability is important to achieving high viral titer. Vector stability is compromised in retroviruses (and the subset of lentiviruses) by an outer envelope consisting of a lipid bilayer interspersed with envelope proteins. There is evidence to support the idea that vector stability, particularly for enveloped vectors, improves at higher media osmolality. Amaral et al. (2008) discovered that enhanced sugar metabolism and the resultant increase in medium osmolality (due to the presence of sorbitol) correlates positively to retroviral stability and productivity. This evidence strongly suggests that a stable retrovirus is heavily dependent on an established media osmolality. Osmolality specifications of media and buffers should account for these effects on vector production and stability, as process repeatability and product yield depend on this process control.