Basic Fibroblast Growth Factor—the Good, the Bad and the Stable
A guest article by the Research & Development team at Thermo Fisher Scientific
What is Fibroblast Growth Factor?
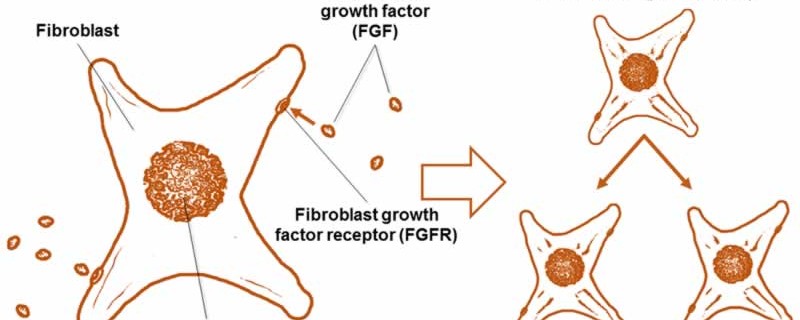
In order to answer this question, first let’s discuss the definition of fibroblast. Fibroblasts are the type of cell responsible for, among other things, fetal development and wound healing. They synthesize the proteins that make up all the tissues, from muscle to skin, in your body. Fibroblast growth factor, or FGF, is a protein that signals fibroblasts to divide (Fig. 1), therefore increasing the population of fibroblasts to better heal injury, for instance. Although FGF was originally discovered by its effect on fibroblasts, it is important to note that FGF also stimulates many other types of cells.
The Good: Basic Fibroblast Growth Factor and Cell Culture
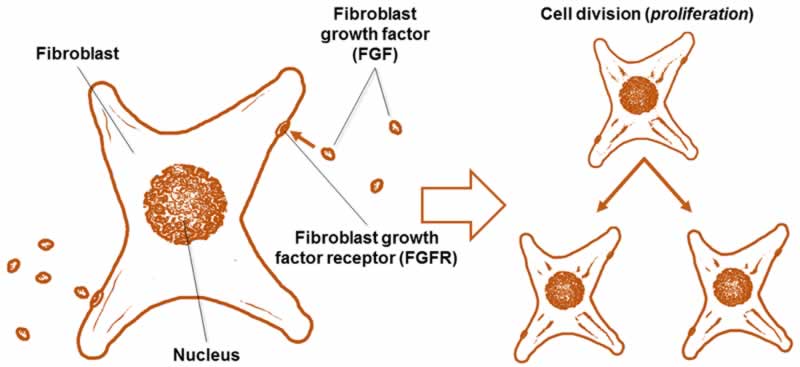
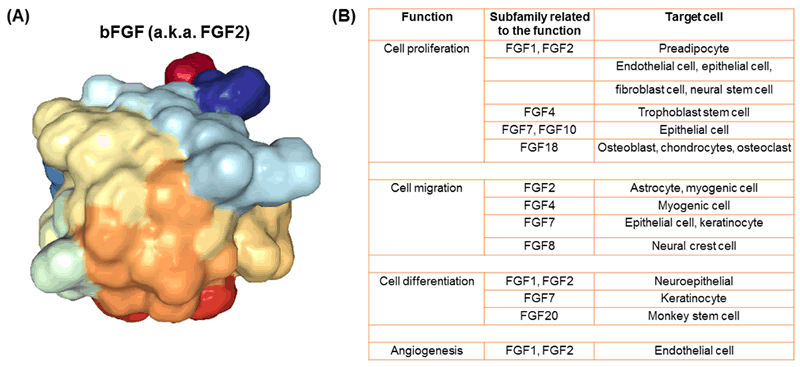
There are many kinds of human FGFs (22 different kinds) [1], but one of the most commonly used in growing cells outside of the body is basic FGF (bFGF, also known as FGF2) (Fig. 2). This protein is widely used as a supplement when growing cells in vitro. Because cells grown on plastic are not nearly as happy as they are in the body, feeding cells bFGF helps signal them to proliferate in order to grow more and more cells. Although bFGF is used to grow many different kinds of cells, one important type is neural stem cells (cells that can differentiate or change into the cells that make up your brain and spinal cord) [2, 3]. Using bFGF to grow neural stem cells allows scientists to research and create treatments for neurodegenerative diseases, like Parkinson’s disease and multiple sclerosis, as well as traumatic brain and spinal cord injuries [2].
The Bad: bFGF is Unstable
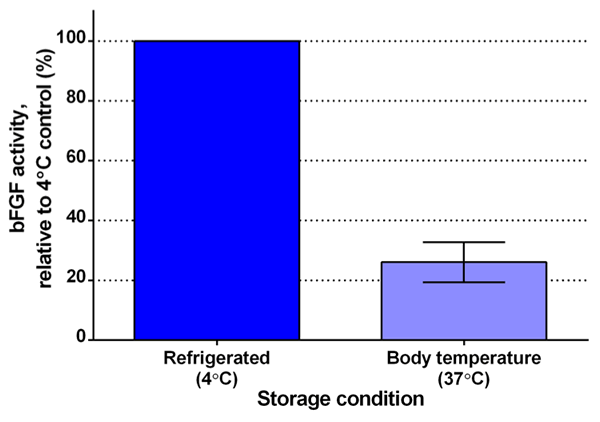
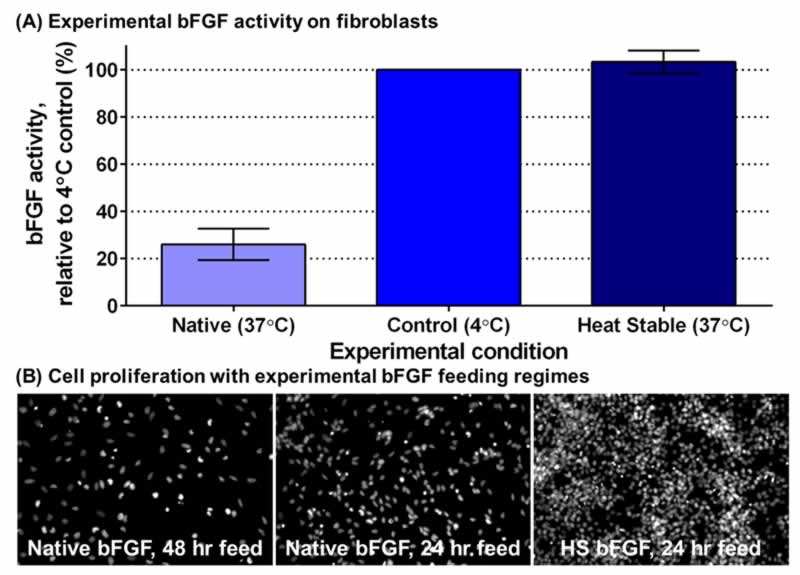
With all the important uses of bFGF for research, you would hope it be easy to use, right? Unfortunately, when scientists feed their cells bFGF and then incubate them at body temperature, the bFGF immediately starts becoming inactivated. You see, bFGF literally can’t take the heat (Fig. 3). When bFGF is at body temperature (which is needed to keep the cells alive and happy), it transforms its structure into a form that cells can’t sense anymore; therefore, the transformed bFGF can’t stimulate the cells and can be considered “inactive.” This means that scientists have to feed their cells fresh bFGF everyday (no weekends!) and, even then, the amount of active bFGF that the cell senses is fluctuating. A constant supply of active bFGF would be much better to keep the cells steadily stimulated and proliferating.
The Stable: Heat Stable bFGF
There is a happy ending to this story! Innovative research has provided a form of bFGF that is stable at body temperature (Fig. 4A). GibcoTM Heat Stable Recombinant Human bFGF has been engineered for greater stability in cell culture conditions, maintaining ≥ 80% bioactivity even after three days at 37°C. Although this Heat Stable bFGF (HS bFGF) is a mutant version of the protein (different amino acid structure than native or typical bFGF), its structure is more than 90% the same and it stimulates cells in the same way [5]. This means that cells are getting a steady supply of the bFGF they need, which leads to more cells with less work from scientists. This allows them to do their important research better and faster.
The value of HS bFGF is best shown with the improved growth of neural stem cells. Even when we fed the neural stem cells native bFGF every 24 hours (the ‘gold standard’) and HS bFGF every 48 hours, we still saw many more cells with HS bFGF (Fig. 4B). This can be attributed to the steady supply of active bFGF that HS bFGF provides; steady bFGF leads to steady proliferation. All of this means that this important protein can now be used more effectively and more efficiently in neurological and other life-saving lines of research.
Please note: GibcoTM Heat Stable Recombinant Human bFGF by Thermo Fisher Scientific is for Research Use Only. It is not for use in diagnostic procedures. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified. For more information about Heat Stable bFGF, including complete data sets on performance and application notes, please go to: http://www.thermofisher.com/heatstablebFGF
Footnotes
-
1. Yun, Y.-R., et al., Fibroblast growth factors: biology, function, and application for tissue regeneration. Journal of tissue engineering, 2010. 2010: p. 218142-218142.
-
2. Martino, G. and S. Pluchino, The therapeutic potential of neural stem cells. Nature Reviews Neuroscience, 2006. 7(5): p. 395.
-
3. Israsena, N., et al., The presence of FGF2 signaling determines whether β-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Developmental Biology, 2004. 268(1): p. 220-231.
-
4. Image from the RCSB PDB (www.rcsb.org) of PDB ID 1BFF (Kastrup, J.S., et al., X-ray Structure of the 154-Amino-Acid Form of Recombinant Human Basic Fibroblast Growth Factor. Comparison with the Truncated 146-Amino-Acid Form. Acta Crystallographica Section D, 1997. 53(2): p. 160-168).
-
5. GibcoTM Heat Stable Recombinant Human bFGF. [cited 2019 January 7]; Available from: http://www.thermofisher.com/heatstablebFGF.