
Boosting iPSC Numbers Using Methodical Optimization of Culture Processes
Human induced pluripotent stem cells (hiPSCs) are invaluable for various applications like drug discovery and disease modeling but require obtaining high cell numbers to be feasible. Achieving these high cell densities are challenging with 2D culture methods, but stirred-tank bioreactors offer a 3D environment conducive to controlling and optimizing growth conditions for hiPSCs.
In a recent application note, “Increasing iPSC Numbers through Systematic Culture Process Optimization”, researchers utilized stirred-tank bioreactors, to provide a 3D culture environment conducive to optimal hiPSC growth conditions. By employing DASbox® Mini Bioreactors and the DASbox Mini Bioreactor System and systematically optimizing process parameters, they achieved a remarkable increase in cell density, surpassing 10-fold compared to uncontrolled conditions, while maintaining stem cell features and viability.
The application note outlines an optimization process using systematic parameter adjustments and in silico modeling. As a result, cell density was increased significantly while still maintaining stem cell properties and viability. The study underscores the importance of bioreactor-based culture methods for advancing stem cell research and therapeutic applications.
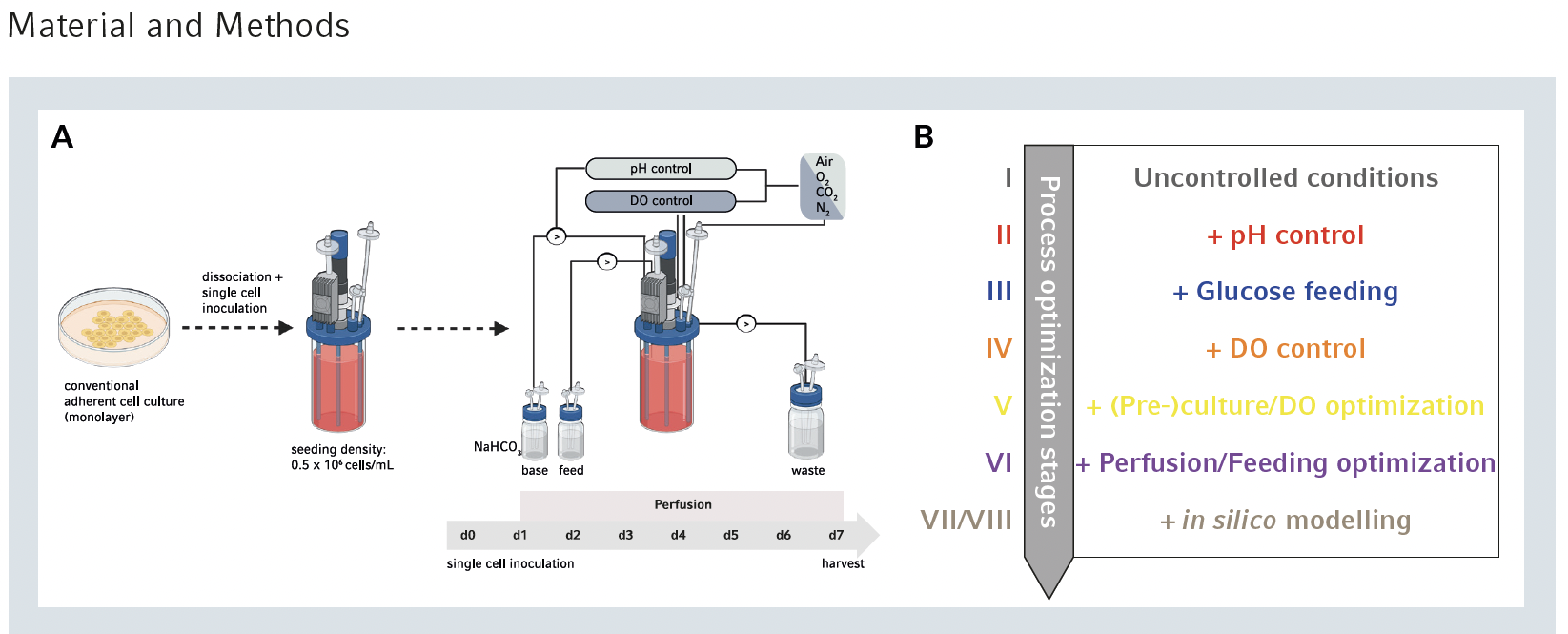
The DASbox Mini Bioreactors and DASbox Mini Bioreactor System were employed for stem cell culturing and optimization. Equipped with an 8-blade impeller optimized for stem cell expansion, the bioreactors facilitated precise regulation of critical process parameters such as pH, feeding rate, and dissolved oxygen (DO) levels. Perfusion operation mode enabled continuous medium flow while retaining cells inside the bioreactor, maintaining constant volume throughout the runs. The system allowed monitoring and adjustment of parameters, with pump and sensor calibration following established protocols. Three different human induced pluripotent stem cell (hiPSC) lines were utilized for the experiments, with pre-culture involving expansion as a feeder-free monolayer culture in flasks under specific conditions, including Essential 8 (E8) medium supplemented with ROCK inhibitor (RI) Y-27623. Medium change intervals were determined based on passaging protocols.
The main experiments involved stem cell aggregate culture without scaffolds for cell attachment. Inoculation of the DASbox Mini Bioreactors was performed using single-cell suspension achieved by detaching the cell monolayer. Different hiPSC lines were inoculated at a viable cell density of 5 × 105 cells/mL in E8 medium supplemented with RI. Cultivation conditions included 37°C temperature, agitation speeds ranging from 60 to 120 rpm, and headspace gassing with 3 standard liters per hour of 21% O2 and 5% CO2. pH control was initiated if the pH dropped to ≤7.0 and maintained at pH 7.0 using CO2 reduction and addition of 1 M NaHCO3. Medium replacement started 24 hours after inoculation at a rate of 150 mL/day, and various compounds such as glucose and Gibco Pluronic F-68 were added during the optimization process. All processes were conducted for 7 days in the bioreactor, with detailed parameters outlined in the application note.
Cell sampling from the bioreactor was done without interrupting stirring, and aggregate formation was monitored using light microscopy. Aggregate size was determined using software, and cell counting was performed after dissociating aggregates into single cells via accutase-treatment. Cell supernatants were stored for subsequent metabolite analyses. Definitive endoderm and intestinal differentiation were conducted after bioreactor expansion, with cells seeded at specific densities and cultured in RPMI 1640 medium supplemented with various factors. Statistical analyses were performed using GraphPad Prism 6 software, with one-way or two-way ANOVA followed by Bonferroni’s post-test used to determine significance. Results are reported as mean and standard error of mean (SEM), with p-values <0.05 considered significant.
Results and Discussion
Influence of pH, glucose feeding, and DO control on hiPSC bioreactor culture
The study utilized the DASbox Mini Bioreactor System to precisely monitor and control pH, glucose feeding, and dissolved oxygen (DO) levels in the culture. pH control was initiated at a setpoint of 7.0, resulting in steady pH values throughout the run and higher glucose consumption compared to uncontrolled conditions. Glucose feeding from day 3 onwards further increased glucose concentrations but also raised lactate levels. DO control with a setpoint of 40% from the first day provided a more stable oxygen supply, although initial DO levels were lower. Different growth parameter settings influenced cell densities, with pH control and glucose supplementation leading to significant increases compared to uncontrolled conditions. However, DO control alone did not substantially improve cell density. The study suggests a compensatory effect of DO supplementation at later timepoints, but further adaptation of the DO strategy is needed to reduce early cell loss and control aggregate size. Overall, controlled pH values and glucose feeding doubled the cell density compared to uncontrolled culture systems, highlighting the positive impact of these parameters on cell growth.
Controlling cell aggregate size and viability by pre-culture optimization, DO adaptation, and agitation adjustment
To address initial cell loss and control aggregate size, a four-stage parameter adaption approach was implemented. Firstly, pre-culture optimization reduced the 2D culture period to 72 hours, resulting in a lower cell confluence. Secondly, a DO-level cascade was introduced, maintaining an initial DO level of 100% before stabilizing it at 40%. Thirdly, the shear protectant Pluronic F-68 was added during inoculation. Finally, agitation speed was increased from 60 to 80, 100, or 120 rpm to control aggregate size. All agitation speed adjustments successfully reduced initial cell loss, with 80 rpm sufficient to maintain aggregate size below 300 μm throughout the run. Despite higher agitation speeds, cell viability remained comparable across conditions. Thus, 80 rpm was chosen to balance aggregate size control and minimal shear forces for subsequent experiments.
Optimized feeding and perfusion rate
To optimize glucose feeding and prevent lactate accumulation, the medium perfusion rate was gradually increased from 1 to 2 culture-volumes/day between days 2 and 5. Simultaneously, glucose concentration was elevated from 3.15 to 6.15 g/L between days 1 and 3, then to 6.15 to 7.65 g/L from day 4 onwards. Despite the expected increase in lactate production, levels remained comparable to non-feeding-optimized approaches. Cell density rose to 18 × 106 cells/mL, nearly double the previous optimization step. Overall, compared to uncontrolled conditions, this comprehensive optimization strategy resulted in a sixfold increase in cell density at day 7 post inoculation compared to uncontrolled conditions.
Further culture optimization by in silico modelling
The precise parameter control provided by the DASbox Mini Bioreactor System and the gentle cell mixing with the 8-blade  impeller resulted in a notable increase in stem cell numbers. To further enhance cell density and reduce wet-lab testing workload, in silico modeling was employed. Using the Berkely-Madonna software, wet-lab results from the previous optimization step were fed into an algorithm to predict further parameter optimization approaches.
impeller resulted in a notable increase in stem cell numbers. To further enhance cell density and reduce wet-lab testing workload, in silico modeling was employed. Using the Berkely-Madonna software, wet-lab results from the previous optimization step were fed into an algorithm to predict further parameter optimization approaches.
Model VII (Table 1) predicted a cell density of 70 × 106 cell/mL on day 7, but actual results reached 23 × 106 cells/mL, surpassing previous approaches. However, an unexpected glucose peak by day 6 indicated the need for further modeling refinement. Model VIII (Table 1) was then developed using wet-lab results from model VII, resulting in a closer match between predicted (40 × 106 cells/mL) and actual (33 × 106 cells/mL) cell densities. These results, confirmed across three different stem cell line cultures, represented almost a 10-fold increase compared to uncontrolled conditions.
Stem cell properties of hiPSCs cultured in a stirred-tank bioreactor
The results demonstrate that process optimization through precise parameter control in a stirred-tank bioreactor enables higher cell numbers while maintaining robust cell viability. Analysis of pluripotency markers TRA-1-60, SSEA-4, OCT-3/4, NANOG, SOX2, and KI-67 confirmed the retention of stem cell properties after 7 days of culture. Additionally, the ability of the stem cells to differentiate into various cell types of the endo-, meso-, and ectoderm germ layers was confirmed through conventional undirected differentiation and specific differentiation protocols. The presence of germ layer-specific markers and the reproducible induction of progenies suggested intact differentiation capabilities. Importantly, no chromosomal abnormalities were observed in the process-derived cells after 7 days of culture. These findings collectively indicate that the pluripotent stem cell population maintained all expected key properties even after cultivation to high cell densities achieved by process optimization in a stirred-tank bioreactor.
In conclusion, the study demonstrates the efficacy of utilizing a controllable and adjustable growth environment for stem cell culture. By employing the precise parameter control capabilities of the DASbox Mini Bioreactor System and systematic adaptation, stem cell densities increased from approximately 3 × 106 cells/mL in an uncontrolled setting to almost 35 × 106 cells/mL, resulting in a total cell number of nearly 5 × 109 cells within the entire culture volume. The 60° pitched 8-blade impeller of the DASbox Mini Bioreactor facilitated efficient mixing, control of aggregate size, and high cell viability while minimizing shear force stress. This step-by-step parameter adaptation approach offers an efficient method for process optimization and development in stem cell culture within bioreactors, providing a roadmap to overcome cultivation bottlenecks and advance stem cell applications.
The data provided in this summary article represents only a fraction of the information available in the application note, please download the full application note for all the data and study details.
For more information, please see Increasing iPSC Numbers through Systematic Culture Process Optimization
All methods and results in this application note are published work by the group of Dr. Robert Zweigerdt, Leibniz Research Laboratories for Biotechnology and Artificial Organs, Hannover