Cell Culture Basics – Mycoplasma 101 – A practical guide to prevention, detection and elimination of mycoplasma contamination
Mycoplasma contamination is a very real concern for research labs. Mycoplasma contamination can compromise cell culture and experiment results, thus wasting valuable resources conducting experiments that ultimately aren’t accurate. Because mycoplasma contamination actually affects the cells’ overall behavior, contamination can result in false interpretation of experimental results and undermines the validity of the resulting data.
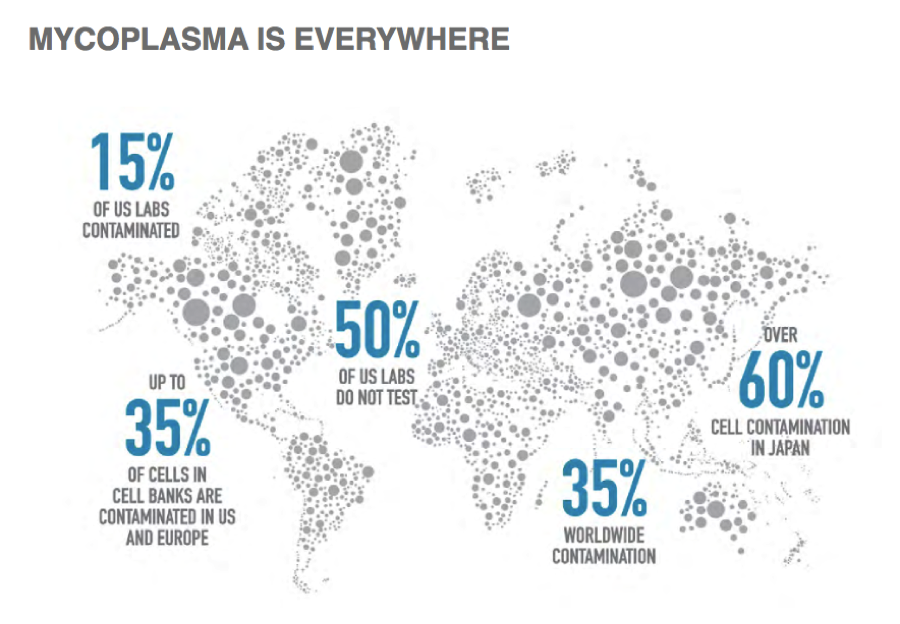
Mycoplasma is huge problem worldwide. A recent mycoplasma infographic, published by Biological Industries USA, shows that the problem is widespread with contamination occurring in every part of the globe.
What is Mycoplasma?
There has been significant discussion in the literature, horror stories really, of years of research pulled into question because of mycoplasma contamination. Not to mention the hundreds of thousands of dollars wasted. So what is mycoplasma and why is it so difficult to detect?
I recently found a webinar hosted by Biological Industries titled, “Mycoplasma 101 – A practical guide to prevention, detection and elimination of mycoplasma contamination,” that did an excellent job outlining what mycoplasma is, why is it so challenging, and what can be done to prevent and deal with contamination.
The webinar, presented by Lia Thornberry Kent, began with a description that mycoplasma is a self-replicating bacteria, the smallest living prokaryote. Because of its size, it can’t be seen under standard microscopes, thus making detection a big challenge. Plus, it does not create any visible signs of contamination – no cloudy media, no cellular build up and no change in pH. It is there, but doesn’t present the usual signs of contamination. The mycoplasma doesn’t overtake the cells or kill them, it lives in the culture and many times goes completely undetected.
Mycoplasma was first detected in cell culture in 1956, so why is it still such an issue? Detection is one challenge, but it is also more difficult to prevent and eliminate than other types of contaminants. It has a pliable membrane that makes it resistant to pressure, temperature, osmolality and dehydration. There are over 180 different species of mycoplasma with over 20 found as contaminants in cell culture. Mycoplasma can infect all types of eukarayotic cells. They have limited metabolic capabilities and they adapt and thrive in a cell culture environment.
Mycoplasma’s Impact on Cell Culture
Mycoplasma compromises cell culture-based experiments in the following ways:
- Competes for nutrients – hinders cell growth and proliferation
- Exposes cells to unwanted metabolites
- Alters levels of protein, RNA, or DNA synthesis
- Changes gene expression, cell signaling and morphology
- Damages membranes and organelles
- Causes mutations and chromosomal changes
Because it often goes undetected, research continues to go on with contaminated cells and these cells are often shared with other labs, where contamination can spread. Due to the impact on the cells, experiments conducted with contaminated cells often can’t be replicated with cells that aren’t contaminated and vice versa. This inability to reproduce results brings research results into question and can negate countless hours of work and research dollars.
Contamination Sources
The most common mycoplasma cell culture contaminates are human, bovine or swine in origin, with human being the largest.
During the webinar, some interesting statistics were presented on how humans introduce mycoplasma that I wanted to share. First, 80.6% of lab techs are carriers and mycoplasma contamination can be spread with a single sneeze or even by talking.
In addition to mycoplasma contamination through human introduction, it can also be introduced through cross contamination with an already infected culture. Poor lab techniques are at fault for much of the cross contamination. Things like using the same media bottles, reusing pipette tips or poor sterile technique can spread mycoplasma from one culture to another.
Mycoplasma Prevention
The next part of the webinar, I found extremely informative with great tips on how to prevent a mycoplasma contamination from happening in the lab.
Lia presented the following tips:
- Always wear personal protective equipment. This includes a lab coat, gloves (be sure to change them often), and a mask if sick or working outside of the hood.
- Know the origin of your cells. Be sure to obtain cells from a reliable source. Mycoplasma survive in liquid nitrogen, so it is important to quarantine new cells and screen them for mycoplasma before introducing them into the lab.
- Good sterile techniques are critical. You must watch for drips from pipettes and clean any spills quickly. It is important to change pipette tips and avoid talking or singing around cells.
- It is important to keep the overall lab space clean. This includes regularly sterilizing work areas. Also it is important to service lab equipment on schedule to ensure all items are working properly.
Pharmacidal™ Spray and solutions to prevent cell culture contamination
One way to help keep the lab clean and to prevent opportunities for contamination is through the use of a Pharmacidal Spray. Pharmacidal Spray offers a powerful prevention solution for disinfecting and sterilizing benchtops and equipment. They prevent the growth of common contaminants such as bacteria, fungi, viruses and mycoplasma.
Biological Industries’ Pharmacidal™ Spray is a disinfectant solution for incubators and work benches in cell culture and molecular biology laboratories in the prevention of mycoplasma. It is more powerful than ethanol and other common lab alcohols for disinfecting benchtops and equipment, yet safe enough even to use on bare hands. Pharmacidal Spray is non-toxic and biodegradable. It is also non-corrosive and can be used on all common work surfaces. BI’s Pharmacidal Spray, when used as directed, has been shown to have no effect on the morphology, cell proliferation and expansion, pluripotency marker expression, or karyotype of human pluripotent stem cells and is appropriate for use in pluripotent stem cell culture.
Also recommended is cleaning the incubator water pan regularly. The water bath is the most prone to contamination; she comically referred to it as a “lab Jacuzzi,” which seemed pretty accurate. Another aid in preventing contamination in these areas is to use a preventative solution that is added to the water. Biological Industries has two solutions, Aquaguard™ solutions 1 and 2 for incubator pans and water baths. The Aquaguard solutions are highly active, non-volatile, non-corrosive, and non-toxic treatment for maintaining water quality and preventing contamination in the water pan of a cell culture incubator.
Aquaguard solutions’ antimicrobial and fungicidal properties are effective against a wide range of common lab contaminants, including gram-negative bacteria, yeast, and fungi. They have been validated for use in incubators and water baths actively culturing sensitive stem cells. Aquaguard 1 Solution, when used as directed, have been shown to have no effect on the morphology, cell proliferation and expansion, pluripotency marker expression, or karyotype of human pluripotent stem cells.
- Working with only one cell line at a time, prevents cross contamination. It is important to only open one bottle of media at a time and only have a maximum of 3-4 culture dishes in the hood at once.
- Don’t use the hood for storage as it can block sterile air flow and will compromise sterility.
- A good practice is to keep the lid on media and culture dishes, and to arrange reagents so you are not reaching over them. Also only lift lids when you are using them.
- Use antibiotics responsibly and sparingly – many common antibiotics don’t work against mycoplasma because it has no cell wall.
- Keep good records. It is important to record any contamination events and keep a log of cells entering and leaving lab. It is also good to track any changes in reagent lots.
- It is important to screen cells regularly. You should screen all new cells as they enter the lab, before and after cells are thawed, any suspicious cell culture, and prior to publication. It is also important to conduct regular monthly monitoring.
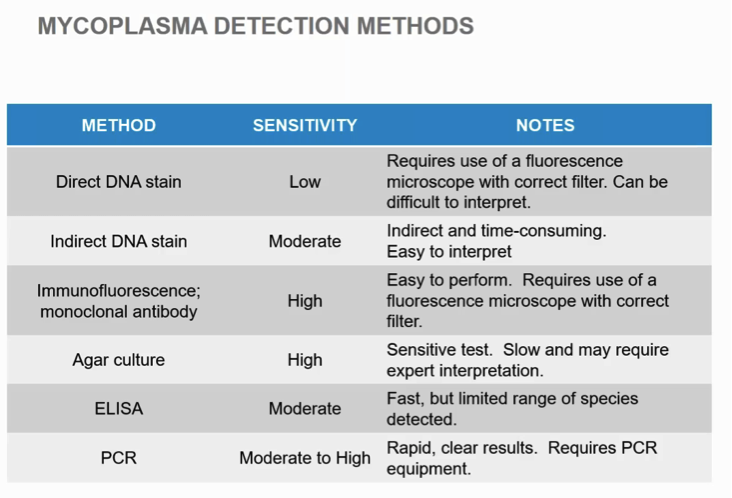
Mycoplasma Detection Methods
After making her point on the importance of screening, Lia discussed mycoplasma detection methods. She presented a slide, which I have shown here that provides an excellent overview of the types of detection methods available and overviews of each.
Direct Agar Culture
She then discussed the various methods in more detail, describing that the direct Agar culture method is the gold standard for detection with the highest sensitivity. It is typically outsourced to specialized labs and takes 3-5 weeks for results. It is the required validation method by regulatory agencies. Direct agar culture, while well-defined and widely accepted, are difficult to administer because they require highly trained personnel to administer and can require up to 28 days for results. As a result, there is a strong desire to find the most effective system for detection.
PCR Test
In response, PCR testing for mycoplasma has been developed. However, this method must be able to prove sensitivity comparable to the traditional methods outlined by the USP and EP and accepted by the regulatory authorities. Biological Industries has developed a PCR test to quickly and accurately test cultures for mycoplasma.
Eliminate Mycoplasma
Lia then goes on to explain how to deal with a positive mycoplasma sample. She said the first thing you should do is retest in-house, then send out for an agar test.
If indeed you have a positive result, you must discard all the cells in culture, disinfect and sterilize the area with bleach and re-test frozen stocks and cells in neighboring incubators.
If the research is such that you can’t discard the cultures, Lia said there is a possibility that you can clean up the cultures with specific antibiotics. However, the efficacy is dependent on the cell line and the level of contamination. Successful recovery after antibiotic treatment is between 65-85%. The antibiotics that have been shown to be successful against mycoplasma are fluoroquinolones, macrolides, and tetracyclines.
Summary
In summary, Lia added that labs need awareness of mycoplasma and a plan in place to prevent contamination. They can do this by establishing lab-wide good cell culture practices and quality control methods.
To attend the April 11, 2017 Mycoplasma 101 Webinar, please visit www.bioind.com or click here to register:
https://attendee.gotowebinar.com/register/7380013685949886721
You may also view an on-demand recording here (https://attendee.gotowebinar.com/register/2516323997094059778)