
Cell Culture Media Analysis for Cell Therapy Applications
 This article was originally published in the eBook
This article was originally published in the eBook“What’s In Your Media”.
You can download all the articles in the series, by downloading the eBook.
In the field of regenerative medicine, cell therapies are increasingly becoming viable therapeutic alternatives to traditional treatments for a wide range of diseases. Most recently, T-cells have been the focus of much clinical investment with chimeric antigen receptor (CAR) T-cell immunotherapies for blood cancers, but other cell types including mesenchymal stem cells (MSCs) and other immune cells (i.e., NK cells, macrophages) have also been investigated for their therapeutic potential. Regardless of the cell type, understanding the best way to culture the cells in vitro to achieve optimal cell activation and expansion, while maintaining functionality is key to the success of these “living medicines”.
One of the keys to success lies in the cell culture media used to culture these cells after isolation. Since each cell type has unique requirements that impact their growth, viability, and functionality, and the cell itself is the therapy additional scrutiny of the media used during cell therapy development is necessary. There is a wide range of cell culture media available from do-it-yourself recipes to commercially available one-size-fits-all complete formulations. Because of the diversity of compositions and a multitude of options for each cell type, it can be a challenging landscape to navigate when identifying the optimal culture media for your cell type.
Additionally, many companies developing cell therapies are looking to lower the very high COGs for current autologous cell therapies by developing allogenic cell therapies which will allow better scalability and standardization but still have safety and efficacy issues. There are rigorous safety and regulatory guidances that restrict certain additives that could pose a health risk to patients, from inclusion in cell culture media. This has resulted in increased investment in developing xeno-free and/or chemically defined media alternatives. Again, highlighting the importance of cell culture media in the development, manufacture and commercialization of any cell-based therapy. Overlooking this aspect and failing to plan appropriately can result in inconsistent performance and quality of the cell therapy and present roadblocks in moving it successfully through regulatory approval. These challenges can ultimately reduce speed to market, which is particularly critical to cell therapies.
Rise Of Metabolomics Analysis To Improve Clinical Success
Unlike traditional biopharmaceutical manufacturing where established, clonal cell lines are utilized, cell therapies using primary and stem cells present some unique challenges particularly in autologous settings. The degree of ex vivo cell expansion, differentiation and functional activation can vary significantly from patient to patient where the starting cells are limited and are often in poor health. Because cell culture media formulations can influence cell characteristics (i.e., growth kinetics, health status and functional properties), there is renewed interest in metabolomics to better understand the impact of various media additives such as glucose, amino acids, vitamins and dipeptides, on critical quality attributes (CQAs) that influence therapeutic efficacy1,2 The effects of metabolism on CAR T-cell efficacy have important implications for improving the quality of T-cells used in adoptive immunotherapy. The potency of therapeutic CAR T-cells has been found to be enhanced by extrinsic factors present in the medium during the manufacturing process3. A thorough understanding of metabolic mechanisms that regulate gene transfer, metabolic fitness, effector function, and persistence may facilitate stepwise improvements in the cell culture media used for ex vivo T cell production. The variety of media formulations intended for therapeutic applications merits comparative metabolic profiling to gain deeper insights into the contribution to exogenous nutrient levels that impact cell metabolism for each specific target.
Another area where metabolic profiling has utility is in the process development stage to inform design of experiment (DOE) optimization of cell culture media formulations to improve overall manufacturing success. Capillary electrophoresis coupled to mass spectrometry (CE-MS) is a rapidly emerging analytical tool for metabolomics investigations to gain more insight into cell culture media, able to separate analytes with high efficiency and at high speed. The at-line CE–MS device, REBEL from 908devices allows rapid quantification of amino acids and vitamins and has the potential to quantify other media components much faster than conventional HPLC-based analytics. Both untargeted methods capable of fingerprinting the complete metabolite profile and also targeted methods enabling the precise and accurate determination of a selected group of metabolites have been developed to analyze the media efficacy4.
As an example, arginine has been identified as a key amino acid for T-cell proliferation and thus its inclusion in media formulations for ex vivo expansion in CAR T workflows is required to obtain the necessary cell numbers for activation and genetic manipulation5,6. However, as shown in Figure 1, a wide range of arginine concentrations can be found across a panel of commercially available media formulations, which can have a significant impact on T-cell performance.
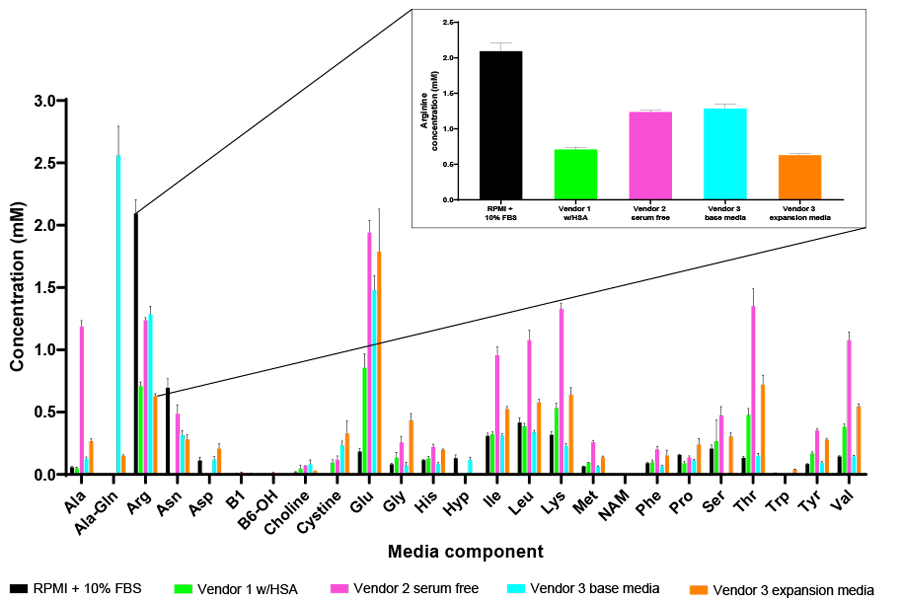
The unprecedented efficacy of CAR T-cell therapies to treat hematologic malignancies has also led scientists to investigate this approach for solid tumor cancers. However, the immunosuppressive tumor microenvironment has proven to be difficult for T-cells to overcome. As such, strategies are being developed to improve T-cell metabolic fitness to overcome the hostile tumor microenvironment (TME) by pre-adapting the cells in vitro in culture media that is more reflective of the oxygen and nutrient status of the TME to prime them towards more effective and robust anti-tumor response once reinfused back into the patient6,7. Metabolic analysis of commercially available T-cell media formulations suggests they are not reflective of physiological conditions, and thus may require fine-tuning to ensure CAR T-cell readiness in vivo.
Similar conclusions have been made with human mesenchymal stem cells (hMSCs) for clinical applications. MSCs are multipotent adult stem cells that have promising therapeutic potential for tissue engineering applications because of their ability to both differentiate into distinctive mesenchymal phenotypes and to induce a regenerative microenvironment by secreting bioactive chemical compounds. However, clinical success has been hampered by poor cell survival and engraftment upon transplantation into the site of injury. The transplanted MSCs experience a hostile, injured microenvironment of ischemia (lack of oxygen and nutrients), inflammation, and oxidative stress, which negatively imposes metabolic stress on the cells, affecting their ability to exert a positive therapeutic effect. Scientists are investigating metabolomics strategies to prime the MSCs in vitro prior to transplantation to improve their survival to increase the overall therapeutic potential of MSCs8.
Fresh Media Analysis
Serum Removal
For clinical applications, there is a shift in the industry to cell media platforms that exclude serum and other animal-derived raw material components to establish more defined growth parameters to improve consistency, mitigate contamination and immunogenic risk, and reduce COGS.
Conventional media used for isolation and expansion often include supplementation with fetal bovine serum (FBS) at 5–20% (v/v). FBS has been a widely utilized media additive because it contains a high levels of nutritional and physiochemical compounds required for attachment and growth. However, safety and regulatory concerns raised by the use of animal serum for clinical use of autologous or allogeneic human blood-derived materials has increased interest in identifying defined serum-free media formulations. Identifying critical factors and their concentrations with an eye towards designing an ideally formulated, defined serum-free medium should be carried out using rational and systematic approaches. In-depth global analysis of serum-containing media can facilitate reformulate efforts to replace serum with more defined components or to provide insight into commercially available, proprietary formulations (Figure 2)9,10. The REBEL has a sensitive dynamic range of 5-100uM of detection to detect low levels of amino acids and other nutrients accurately, without interference from serum proteins, unlike other quantitative methods.
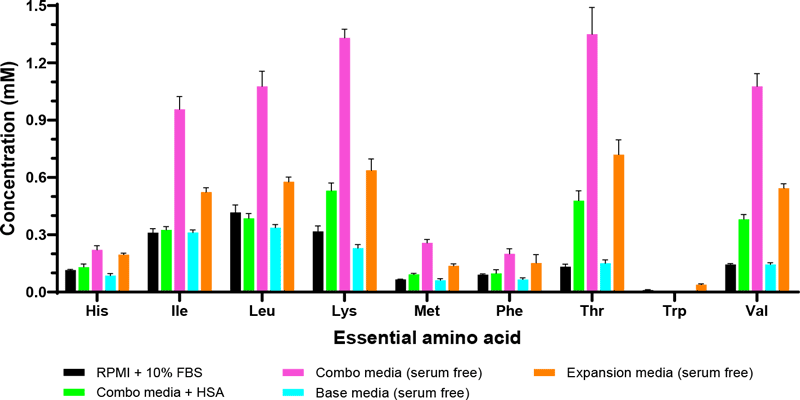
Implementing defined media formulations into cell therapy manufacturing would enhance consistency across cell bioprocessing protocols with the provision of a more uniform and controllable environment, which may be crucial to boost clinical efficacy.
Media Optimization
Another reason to focus on media development is to optimize the formulation to maximize the expansion of your cell type to increase output while reducing cost of goods (COGS). In an autologous setting, each patient’s health status and demographics translates to a unique cell population, which imparts variability to the subsequent cell therapy manufacturing process emphasizing the importance of in-process controls to ensure a consistent drug product is produced in each and every manufacturing run even in the presence of such variability. Process analytical technologies (PATs) can provide visibility into and information on critical quality attributes (CQAs) throughout the manufacturing process and QC release testing where speed, accuracy and throughput are needed to provide real-time process feedback for optimization efforts and to speed product release. The focus of PAT within the Quality by Design (QbD) framework has highlighted a need for better analytical tools that can allow rapid and frequent monitoring to inform process development in real-time. Current analytical methods suffer from long lag time between sampling and data generation ranging from days to weeks, which is prohibitive to effective design of experiment (DOE) methodology. Additionally, these assays may lack the resolution needed to show subtle changes that may result from incremental process optimization steps.
Spent Media Analysis
Targeted metabolomics-based PAT allows for metabolic profiling of the T-cells throughout the manufacturing process through spent media analysis. Particularly with the adoption of more automated and closed processes for large-scale commercial manufacturing, analytical tools that can be operated at- or on-line to minimize manual handling time are desirable. Traditionally, groups involved in process development do not have access to the necessary analytical tools or resources required for this type of analysis. Outsourcing analysis to core labs or CROs can take days to weeks from sample-to-result, which can severely delay process development and PAT implementation efforts.
The REBEL is built for purpose, enabling frequent, at-line measurements from the bioreactors for active, near real-time monitoring of CQAs, which has direct implications on the therapeutic efficacy and safety, to ensure product quality and batch-to-batch consistency. Its small, self-contained footprint fits alongside bioreactors and has the ability to rapidly analyze samples to provide quantitative identification and analysis on a panel of >30 analytes including all the key amino acids, biogenic amines, water soluble vitamins and dipeptides with accuracy and reproducibility (Figure 3). Integrating data reporting capabilities with portable report formats and automated performance qualification and 21 CFR Part 11-compliant software make the REBEL easy to integrate into cGLP/cGMP environments.
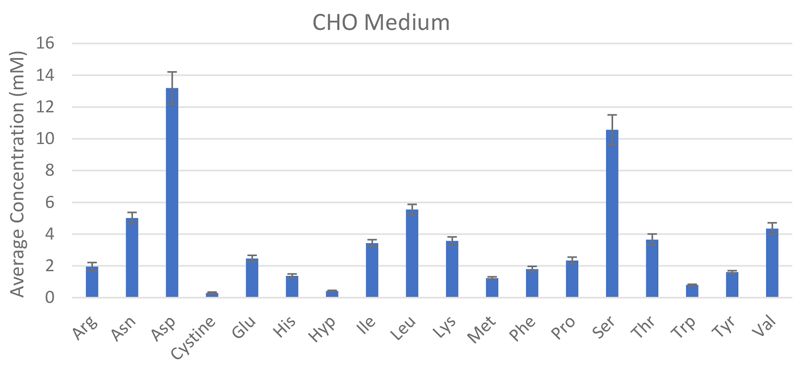
Scale out and scale up are two paradigms to increase production for cellular therapies, each with their own distinct challenges. Scaling out involves increasing the number of operation units in parallel to increase capacity whereas, scale up increases the size of the operation unit, which typically means moving to larger culture vessels that are different than what was used in small-scale production. Scale out options are easy to implement since the culture parameters do not change but inefficiencies in the scale out model may result in increased COGS that doesn’t meet long-term product needs. During scale up to larger bioreactor platforms, the change in vessel size, agitation and other physiological parameters can affect growth and cellular performance, which can require extensive process optimization to ensure that CQAs are still met. In some cases, it may be necessary to select a different cell culture media or the addition of additional factors to maintain phenotype control and optimize cell growth without inducing other changes that could result in a loss of functionality.
A Perspective On Outsourcing
In efforts to bring therapies to market sooner, many companies partner with CMOs and CDMOs to aid in the movement of their cell therapy products through all phases of clinical development. However, with regards to outsourcing, the developer may have less oversight over the manufacturing process, and disruptions at the CMO or CDMO can create bottlenecks for the developer, particularly if they rely on a single CMO or CDMO to fulfill their needs11. Because many of the current generation of regenerative medicine products are unique and highly specialized, requiring customized components and non-standard technology approaches that need specially trained staff, traditional CMOs/CDMOs struggle to adapt to these bespoke requirements. This can ultimately delay tech transfer and extend timelines that negatively impact the developer and their patient population especially when speed to market is so important. Additionally, industry experts recommend that every developer should keep some aspect of manufacturing in-house even when partnering with a contract organization for certain aspects of the manufacturing process to build process knowledge and understanding, advantageous when seeking regulatory approvals12.
New analytical platforms like the REBEL have broad utility across cell therapies from development through to commercial manufacture. The speed and throughput of CE–MS based approaches to deliver in-depth spent and fresh media analysis can be leveraged to improve the process understanding and efficiency and maintain visibility and control throughout all stages of cell therapy production with the ultimate goal of bringing these life-saving therapies to patients faster.
Footnotes
-
1. Zang L, Frenkel R, Simeone J, Lanan M, Byers M, Lyubarskaya Y. Metabolomics profiling of cell culture media leading to the identification of riboflavin photosensitized degradation of tryptophan causing slow growth in cell culture. Anal Chem. 2011;83(13):5422-5430. doi:10.1021/ac2009492
-
2. DePalma A. Better Cell Culture through Metabolomics. Genetic Engineering & Biotechnology News. July 1, 2015. https://www.genengnews.com/magazine/252/better-cell-culture-through-metabolomics/.
-
3. Ghassemi S, Martinez-Becerra FJ, Master AM, et al. Enhancing Chimeric Antigen Receptor T Cell Anti-tumor Function through Advanced Media Design. Mol Ther Methods Clin Dev. 2020;18:595-606. doi:10.1016/j.omtm.2020.07.008
-
4. Klupczynska A, Misiura M, Miltyk W, et al. Development of an LC-MS Targeted Metabolomics Methodology to Study Proline Metabolism in Mammalian Cell Cultures. Molecules. 2020;25(20):4639. doi:10.3390/molecules25204639
-
5. Geiger R, Rieckmann JC, Wolf T, et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell. 2016;167(3):829-842.e13. doi:10.1016/j.cell.2016.09.031
-
6. Wei J, Raynor J, Nguyen TL, Chi H. Nutrient and Metabolic Sensing in T Cell Responses. Front Immunol. 2017;8:247. Published 2017 Mar 9. doi:10.3389/fimmu.2017.00247
-
7. Xu X, Gnanaprakasam JNR, Sherman J, Wang R. A Metabolism Toolbox for CAR T Therapy. Front Oncol. 2019;9:322. Published 2019 Apr 30. doi:10.3389/fonc.2019.00322
-
8. Salazar-Noratto GE, Luo G, Denoeud C, et al. Understanding and leveraging cell metabolism to enhance mesenchymal stem cell transplantation survival in tissue engineering and regenerative medicine applications. Stem Cells. 2020;38(1):22-33. doi:10.1002/stem.3079
-
9. Medvec AR, Ecker C, Kong H, et al. Improved Expansion and In Vivo Function of Patient T Cells by a Serum-free Medium. Mol Ther Methods Clin Dev. 2017;8:65-74. Published 2017 Nov 7. doi:10.1016/j.omtm.2017.11.001
-
10. Jung S, Panchalingam KM, Rosenberg L, Behie LA. Ex vivo expansion of human mesenchymal stem cells in defined serum-free media. Stem Cells Int. 2012;2012:123030. doi:10.1155/2012/123030
-
11. Lehmicke M. Manufacturing Cures: Infrastructure Challenges Facing Cell And Gene Therapy Developers. Pharma Intelligence | Informa. June 10, 2019.
-
12. Harris E. The Case For In-House Manufacturing Of Cell Therapies. Cell & Gene. August 18, 2020 https://www.cellandgene.com/doc/the-case-for-in-house-manufacturing-of-cell-therapies-0001.