Cell Therapies Require Well-defined, Optimized Media to Enable Efficient Transition Between Research, Clinical and Manufacturing Scale Culture
The ongoing success of cell therapies in the clinical setting has put an increased focus on optimizing the manufacturing process for these therapies. In particular, cell expansion for clinical use is critical and requires a highly optimized media appropriate for clinical and commercial manufacturing. Increasingly the industry is moving towards using well-defined media without the addition of serum. In addition, clinical and ultimately commercial manufacturing requires a scalable process. This includes a medium that enables large-scale manufacturing systems, such as microcarrier-based suspension culture platforms. To meet these needs, MilliporeSigma has published an article, which describes the use of their Stemline® XF MSC Medium in conjunction with the 3L Mobius® Stirred Tank Bioreactor to increase yield and functionality. The Stemline® XF MSC Medium was designed for efficient expansion in planar and microcarrier-based culture platforms, easing the transition between research, clinical, and manufacturing scale culture.
We were fortunate to be able to include the full article below and an interview with Kathleen Ongena, Ph.D., Head of Customer Applications, Cell Therapy Bioprocessing.
Stemline® XF MSC Medium has High Yield and Functionality in the 3 L Mobius® Stirred Tank Bioreactor
Abstract
Optimizing ex vivo cell expansion processes in preparation for clinical use is a critical step in cell therapy manufacturing. Given the curative and lifesaving impacts these therapies can have on patients, performance and quality are essential outputs of any expansion process. Here, we discuss the performance of Stemline® XF MSC Medium, which promotes expansion of human mesenchymal stromal/ stem cells (hMSCs) to high densities while maintaining cell identity and quality. This product was designed for efficient expansion in planar and microcarrier-based culture platforms, easing the transition between research, clinical, and manufacturing scale culture. We describe the ex vivo expansion of bone marrow-derived hMSCs cultured in Stemline® XF MSC Medium in a 3 L stirred tank bioreactor-microcarrier platform.
Introduction
The long-term outlook for stem cell therapy predicts a shift toward more defined media and high-quality raw materials. Currently, fetal bovine serum (FBS) is still used in the majority of MSC products being developed for clinical use. For many reasons, cell therapy developers are being encouraged to move away from the use of serum at earlier stages of product development1. Alternative formulations include solutions often categorized as “serum free,” “xeno free,” or “defined.”
Developing a well-defined, scalable bioprocess is also important to produce robust and safe cell therapies2. To support that vision, there is an increased need for cell therapy reagents compatible with microcarrier-based suspension culture platforms. This would allow for both small-scale studies and large-scale therapeutic manufacturing, without the need to redefine critical reagents when entering a new phase of the therapy development process.
Pre-clinical and clinical level data will be essential in determining the safety and therapeutic effects for different disease indications for hMSC therapies3. Thus, it is extremely beneficial to investigate serum-alternative media formulations that support high performance expansion and are compatible with scalable manufacturing processes. Here we detail the process parameters and growth results of bone marrow-derived hMSCs cultured in Stemline® XF MSC Medium in a 3 L stirred tank bioreactor-microcarrier platform.
Methods
Stemline® XF MSC Medium, consisting of Stemline® XF MSC Basal Medium (Cat. No. 14371C) and Stemline® XF MSC Supplement (Cat. No. 14372C), was used in our 3 L Mobius® bioreactor system with minimal process modifications.
Bone marrow-derived hMSCs were scaled up in either Stemline® XF MSC medium, alpha-MEM supplemented with 5% human platelet lysate (hPL), or a commercially available xeno-free competitor medium. Each medium was supplemented with L-glutamine at a final concentration of 2 mM. Shear protectants were added to each medium. Collagen Type I-coated microcarriers were chosen for hMSC expansion.
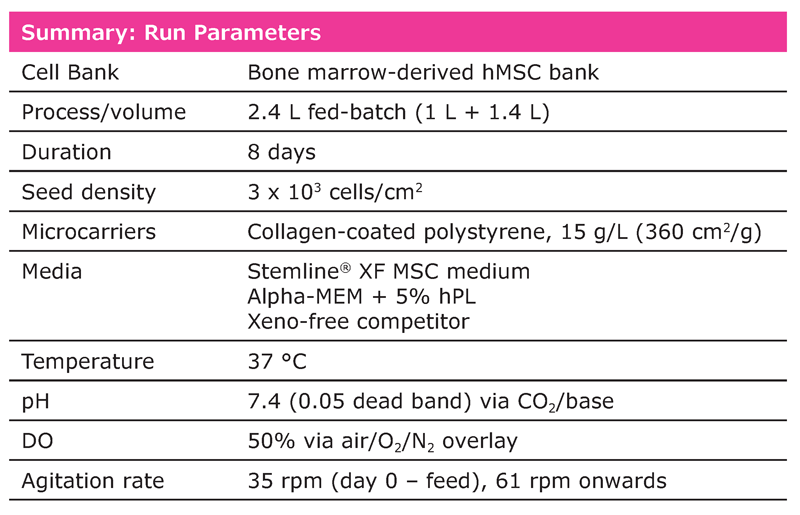
Over the duration of the run, dissolved oxygen (DO) and pH were continuously monitored and controlled at 50% and 7.4 respectively. Nutrient and metabolite levels were measured daily (BioProfile® FLEX2, Nova Biomedical). The L-glutamine and glucose concentrations were restored after they dropped below 50% of the initial levels. Total cells were determined by manual sampling and counted in triplicate.
The hMSCs were harvested on day 8 of the bioreactor culture. Expression of the surface markers CD44, CD73, CD90, CD105, CD11b, CD14, CD19, CD34, CD45, CD79a, CD106, CD274 and HLA-DR was assessed by flow cytometry. Potency of the cells expanded in the Stemline® XF MSC medium was also determined. After bioreactor expansion, the hMSCs were re-plated in tissue culture flasks and activated (licensed) by supplementing the media with 25 ng/mL (final) of tumor necrosis factor (TNF)-α and interferon (IFN)-γ. After three days, the expression of the immune potency-related surface markers CD274, CD54, HLA-DR, CD80, CD40, and CD86 was determined by flow cytometry. The indoleamine 2,3-dioxygenase (IDO) activity of the activated hMSCs was also assessed by measuring the concentrations of L-tryptophan and L-kynurenine in the cell culture supernatant. Trilineage differentiation capability was tested using AdipoMAX Differentiation Medium (Cat. No. SCM122-1KT), ChondroMAX Differentiation Medium (Cat. No. SCM123) and OsteoMAX-XF Differentiation Medium (Cat. No. SCM121).
Results
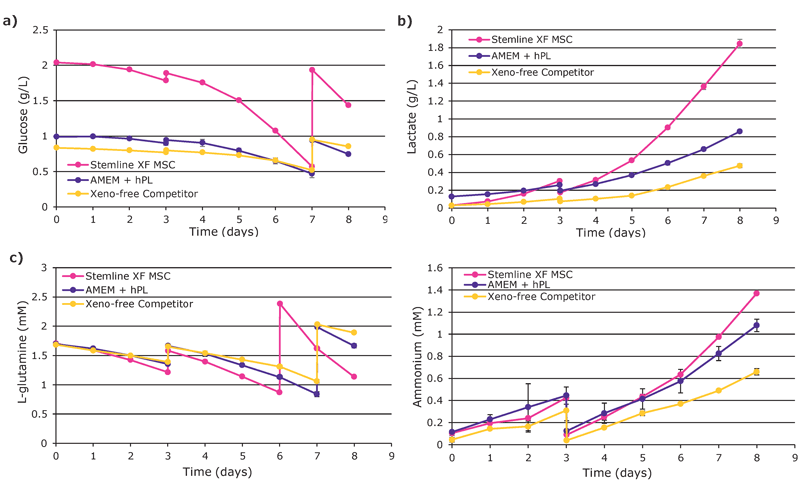
Overall, hMSCs expanded in the Stemline® XF MSC medium showed superior growth performance (Figure 1). After an eight-day culture, a yield of 8.3 E+08 total viable cells was attained with the Stemline® XF MSC medium, followed by 5.25 E+08 cells with AMEM + hPL, and 3 E+08 with the xeno-free competitor medium. The cumulative population doublings were 5.6 with the Stemline® XF MSC medium, 5.0 with AMEM + hPL, and 4.2 with the competitor xeno-free medium.
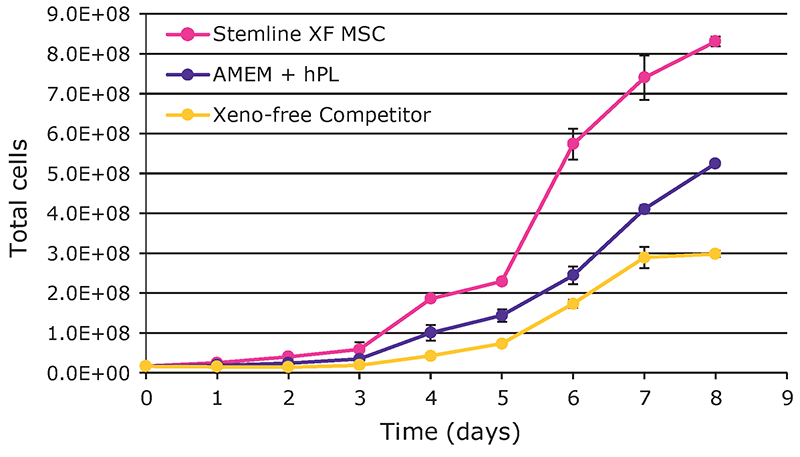
pH and DO remained within control constraints with notable spikes on Day 3 during addition of media and microcarriers (data not shown). Nutrient depletion and waste production in Stemline® XF MSC medium were increased compared to other media and are consistent with the higher growth rates (Figure 2).
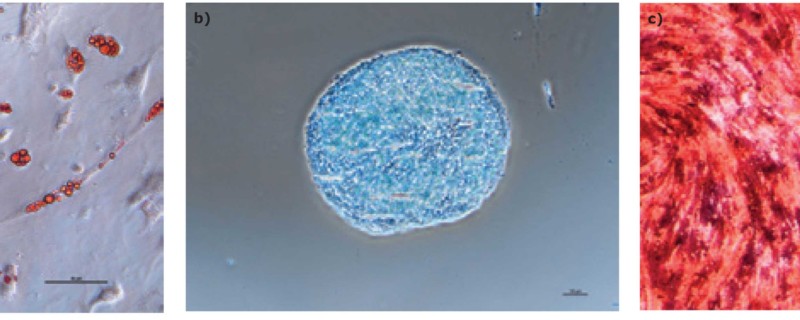
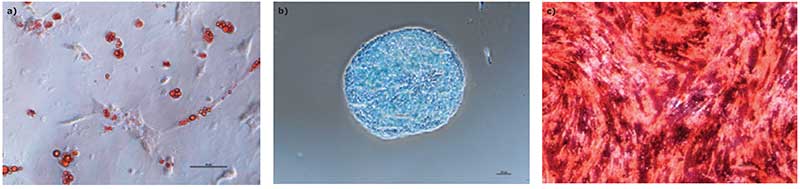
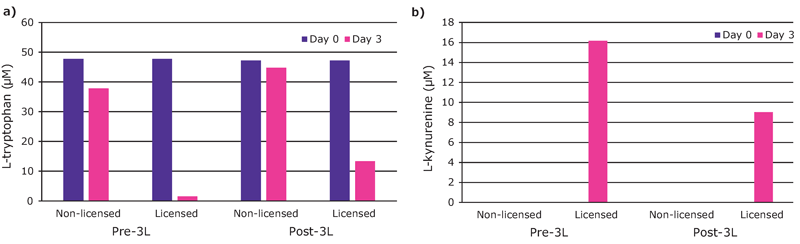
We found that hMSCs expanded in Stemline® XF MSC Medium retained typical identity markers and potency after 3 L bioreactor culture. The cells highly expressed CD44, CD73, CD90, and CD105, concomitant with little to no expression of CD11b, CD14, CD19, CD34, CD45, CD79a, CD106, CD274 and HLA-DR (Figure 3). The hMSCs successfully differentiated into adipocytes, chondroblasts, and osteoblasts as shown by positive stains of lipid vacuoles with Oil Red O, glycoconjugates with Alcian Blue, and calcium deposits with Alizarin Red respectively (Figure 4). Several MSC surface markers related to immune function including CD274, CD54, and HLA-DR were upregulated after a three-day incubation with TNF-α and IFN-γ (Figure 5)4. There was increased IDO activity with licensed hMSCs which was confirmed through the significant depletion of L-tryptophan and production of L-kynurenine (Figure 6).
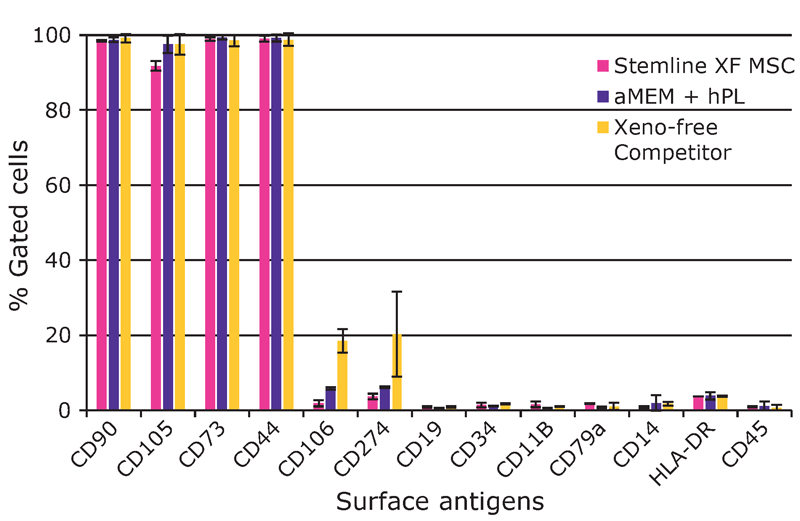
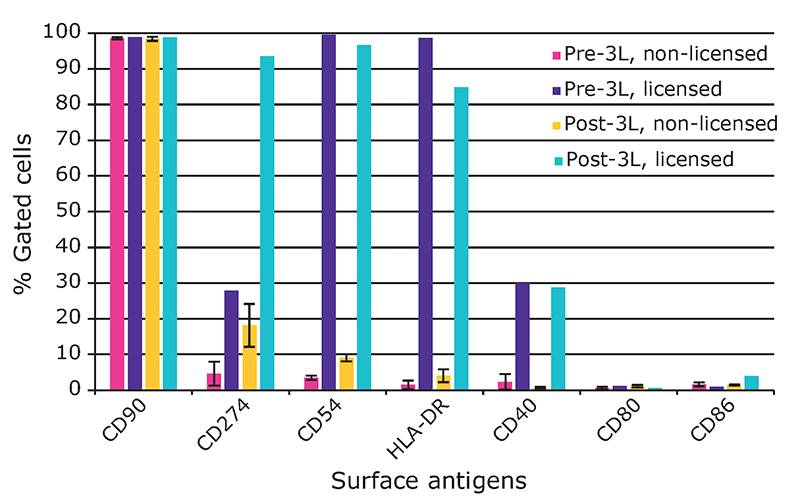
surface antigen levels after expansion in the 3 L bioreactor. There was increased expression of CD274, CD54, HLA-DR, and CD40. The marker CD90 was included as a control.
Discussion/Summary
The Stemline® XF MSC medium consistently outperformed alternative xeno-free formulations with 3 L bioreactor culture. The increase in hMSC growth rate in this media has obvious implications for manufacturing strategy, potentially allowing new products to have shorter expansion timelines and increasing savings on reagents, materials, and labor. The cells expanded in this medium also retained prototypical MSC identity markers and immunomodulatory capabilities after 3 L bioreactor culture.
Our data demonstrates the utility of Stemline® XF MSC Medium for bench-scale bioreactor expansion of hMSCs in microcarrier-based cultures. High yields of functional hMSCs were generated when compared with other xeno-free alternatives. High quality media and supplements are positioned to dynamically impact cell manufacturing processes and will enhance the development of the burgeoning cell therapy field.
Interview with Kathleen Ongena, Ph.D., Head of Customer Applications, Cell Therapy Bioprocessing
Were other microcarriers considered for this study aside from collagen type I?
This study was performed with collagen type I because it was historically used as a control. In more recent studies, we are evaluating different xeno-free microcarriers.
Were there any differences observed with cells cultured in Stemline XF MSC medium when it came to releasing the cells from the microcarriers for downstream differentiation compared to traditional formulations?
Harvest yields with the Stemline XF MSC medium were 30% higher than AMEM + hPL and 45% higher than yields with the commercially available xeno-free medium. These numbers trend with the overall growth profiles. Interestingly, MSCs most easily dissociated from the microcarriers taken from the Stemline cultures. There were numerous microcarrier–cell aggregates particularly from the AMEM + hPL cultures that did not dissociate. This resulted in reduced harvest efficiency as compared to the Stemline cultures.
Is it recommended for researchers interested in hMSC culture to start with this formulation so that they won’t need to transition from FBS-based media when moving to bioreactor culture from 2D planar cultures?
Yes, it is recommended to start the seed train in the Stemline XF MSC media formulation as described in this tech note. Ideally, the MSCs are derived or isolated from their tissue source in the Stemline XF MSC media.
Can hMSCs be isolated in Stemline XF media?
We have isolated MSCs from bone marrow aspirates in our medium using different types of xeno-free coatings and surfaces. We are planning to further investigate the different conditions and share our findings
Can you speak to the performance of Stemline XF for hMSC culture on conventional 2D planar technology?
We have seen robust performance with the Stemline XF MSC media in 2D planar culture. Although not always needed, some cell lines will benefit from a specialized hydrophilic surface or xeno-free coating.
Since the doubling rate of the cells is significantly faster, the cells spend less time in culture compared to a media formulation where it takes a longer culture time to achieve the same number of cells. Can you speak to the benefits of this for downstream applications?
The major benefit of a shorter culture duration is a more readily available product. The downstream processes of harvest, concentration, and final fill should not be affected by culture time particularly if the cell properties remain constant. For therapeutic applications, some studies have shown reductions in MSC potency as well as accumulation of genetic abnormalities with extended time in vitro. If these phenomena are correlated with culture duration instead of the number of cumulative doublings, a medium supporting faster growth is certainly beneficial.
Has this strategy been utilized with MSCs derived from other sources – i.e. Wharton’s Jelly – umbilical cord, induced pluripotent stem cell-derived MSCs?
The strategy or process described in this technical note was developed with bone marrow derived MSCs. When using a different kind of MSC or stem cell, some optimization of the process might be required.
Footnotes
-
1. Karnieli, O., et al. A consensus introduction to serum replacements and serum-free media for cellular therapies. Cytotherapy. Feb;19(2):155-169
-
2. Panchalingam, K., et al. Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: a review. Stem Cell Res Ther. 2015 Nov 23;6:225.
-
3. Lukomska, B., et al. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019 Apr 9;2019: 9628536.
-
4. Galipeau, J., et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016 Feb; 18(2): 151–159.