Cellvento® 4HEK Medium Provides a Comprehensive Media Solution for all Scales of AAV Production
Streamlining the adeno-associated virus (AAV) production process is a top priority for gene therapy development, as there are still issues that limit consistency and productivity. When looking at the AAV production process using human embryonic kidney (HEK) 293 cells, cell culture media has been a challenge. Specifically, there are a limited number of serum-free media that are specifically designed for AAV production at all scales, and this impacts the production consistency. To further complicate matters, there are a variety of commercially available HEK293 cell lines used and each have unique properties and different nutritional requirements, which make it difficult and time consuming to find a medium to support each cell line. In addition, because of the lack of a stable production cell line, plasmids are used for transient transfection, which means a transfection friendly media is important. Lastly, high AAV titer is needed due to large dose requirements, this means that a successful medium must be extensively optimized to each cell line to ensure high productivity.
Cellvento® 4HEK Medium
To solve these issues, MilliporeSigma recently launched Cellvento® 4HEK Medium, a chemically defined and animal component free medium optimized for AAV production in multiple HEK293 lineages. Cellvento® 4HEK Medium has been streamlined and cell culture media components incorporated to increase medium stability and reduce protein oxidation. Cellvento® 4HEK Medium supports consistent performance from R&D to manufacturing and reduces quality and regulatory qualification burdens.
In a recent study, MilliporeSigma tested AAV2 serotype production in four different HEK293 cell lines cultivated in five different media. Cells were adapted in medium for at least three passages, then cells were diluted to 1×106 cells/mL and transfected at day three with PEI transfection reagent and transfection efficiency was measured by flow cytometer 48 hours post transfection (Figure 2). Harvest was conducted 72 hours post transfection and AAV2 full capsid titer (genome concentration) was measured by droplet digital PCR (ddPCR) (Figure 3).
Robust Growth
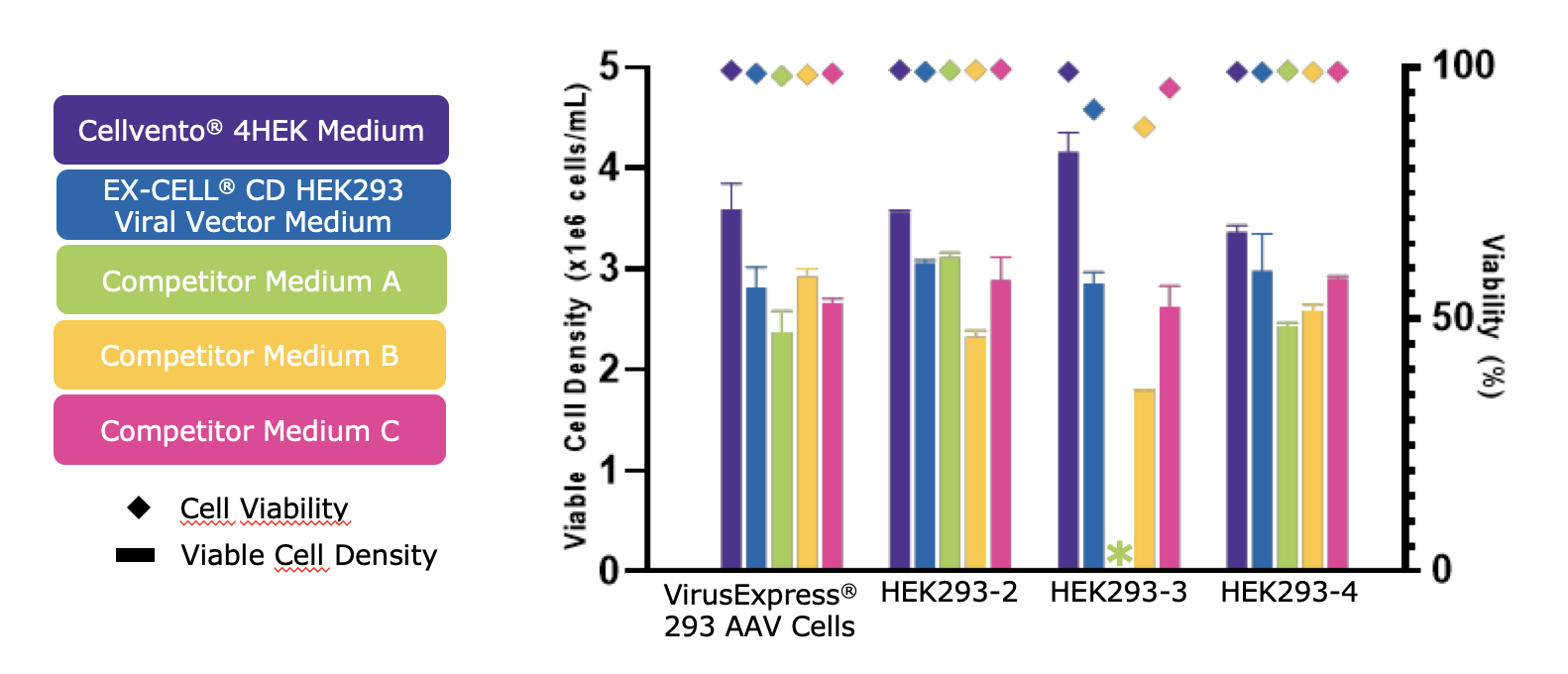
The data demonstrated robust growth using Cellvento® 4HEK Medium across multiple cell lines (Figure 1). When comparing viable cell densities and viability at Day 3, Cellvento® 4HEK Medium provided high viable cell density and viability across all HEK293 cell lines tested. Robust growth data across different cell lines tested confirms that Cellvento® 4HEK can be used successfully in multiple HEK293 lineages.
One Medium Formulation through Entire Process
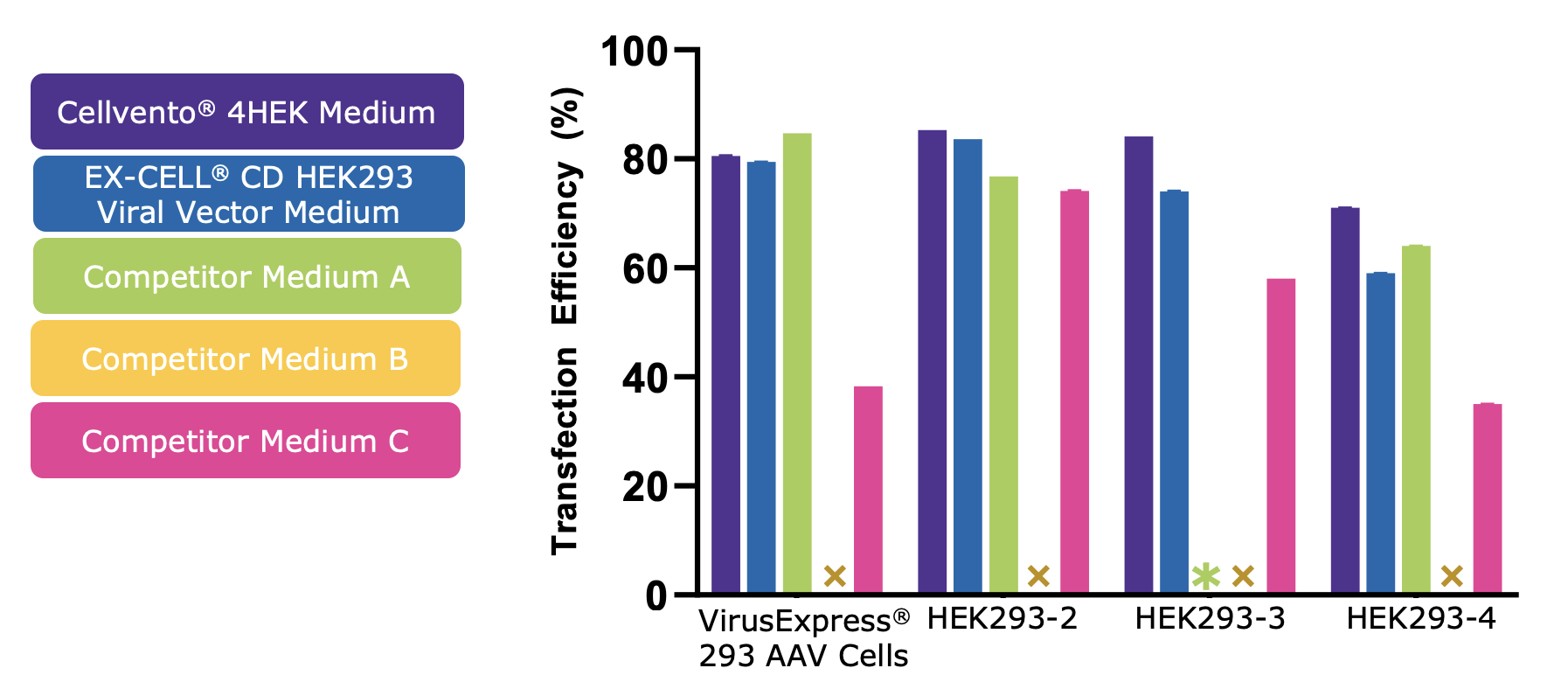
Cellvento® 4HEK Medium can be used throughout the entire production process. It is compatible with common transfection reagents and can therefore be used from growth to production with no need for feed or media exchange. In the same study, when transfection efficiency was compared across the 5 media tested, Cellvento® 4HEK demonstrated a high level of PEI-mediated transfection in all cell lines (Figure 2).
High AAV Titer
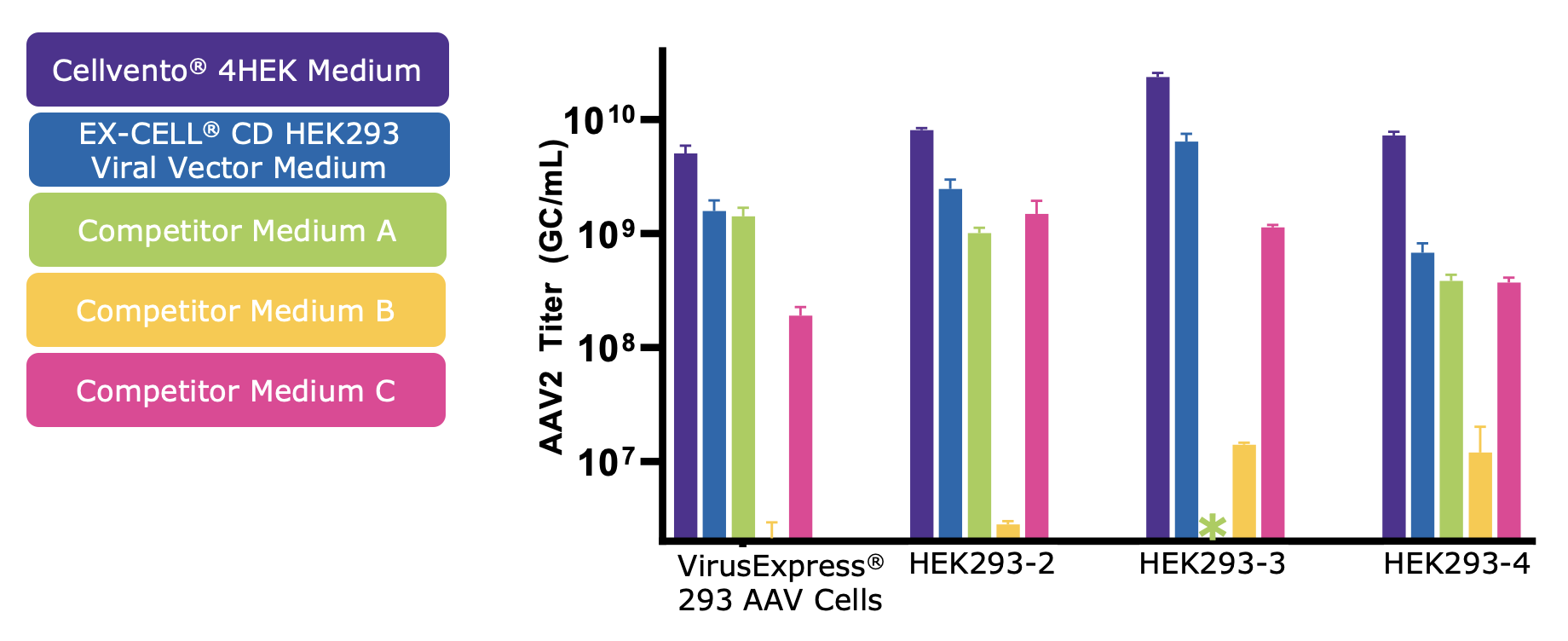
Cellvento® 4HEK Medium has been optimized for high AAV titers in multiple HEK293 cell lineages. AAV2 genomic titer was measured across media and 4 HEK293 cell lines. Cellvento® 4HEK Medium produced the highest titer production performances and the second best is another chemically defined medium from the MilliporeSigma, the EX-CELL® CD HEK293 Viral Vector Medium. (Figure 3). The AAV titer results presented in Figure 3 represent results with no transfection optimization. After selecting the best medium to use, AAV titer can then be improved with transfection optimization (data not shown).
Cellvento® 4HEK Medium Development
I was fortunate to be able to talk with MilliporeSigma experts about the development of Cellvento® 4HEK Medium and how they were able to optimize the medium for multiple cell lines and scales of production. They explained that there is a lack of a stable producer cell line for AAV production, thus the use of plasmid for transfection is required. However, specific raw materials found in many cell culture media formulations are known to interfere with the most common transfection reagent PEI. These specific raw materials had to be removed from the medium to enable efficient transfection, which is why with other cell culture media, the medium has to be removed and a different media used for transfection. That is not the case with Cellvento® 4HEK Medium where transfection efficiency, growth, and productivity are optimized, enabling the same medium to be used during all phases of production.
The MilliporeSigma team of media development experts screened multiple and diverse media with several HEK293 cell lines and measured the AAV titer. Next, they identified the critical components of the media using a multivariate analysis to look for components that increased viable cell density and productivity of the different cell lines. Then they developed a design of experiments (DoE) approach combining data obtained previously with studies using multiple commercially available transfection reagents and bioreactor parameters during the growth period. Transfection and production were evaluated separately to provide the most information for customers to help them save time in that process. This approach allowed their team to reach the highest viable cell density and AAV titers without the customers having to face the bottleneck of a non-optimized step in the AAV production process.
Technical Support
In addition to the extensive work that went into developing the medium, MilliporeSigma is committed to supporting customers throughout the production process. They offer a multi-disciplinary team that brings together a unique combination of expertise and experience required to anticipate challenges and to help customers reach their objectives.
To learn more, please see Cellvento® 4HEK Medium