
CHOSOURCE™ GS Knockout Cell Line Provides Flexible Solutions to Biomanufacturing Challenges
Biomanufacturing faces growing difficulties derived from increasingly complex design of therapeutic molecules, the need to shorten time to market, and pressure to reduce the cost of drugs. Chinese hamster ovary (CHO) cells have long been the workhorse of the industry because of their adaptability and robustness. However, there is still a need to keep optimising these CHO cells to meet evolving biomanufacturing challenges by engineering cell lines that are more robust and flexible, provide higher yields and facilitate the control of product quality attributes.
In addition, recent years have witnessed a renaissance in the use of perfusion and process intensification technologies with diverse motivations, ranging from better controlling product quality (particularly in labile products), to increasing yield for a given footprint, or reducing timelines and costs. These diverse manufacturing paradigms have given rise to flexible facilities capable to handle diverse molecular types requiring different production workflows, and production scales without requiring substantial engineering adaptations.
CHOSOURCE GS KO Cell Line
The first requirement to achieve a robust and flexible manufacturing platform is a flexible expression cell line (CHO) capable of expressing diverse therapeutic product architectures and is able to be used in different scales and process workflows in order to deliver adequate product yields and quality in the required timeframes and costs. In recent years, cell line engineering efforts have generated improved host cell lines for therapeutic production that maximize protein expression and robustness in bioproduction. One example is Horizon Discovery’s CHOSOURCE™ GS knockout cell line, using recombinant adeno-associated virus (rAAV) gene editing technology. The elimination of the endogenous glutamine synthetase (GS) gene facilitates the selection of highly expressing clones with optimal performance during cell line development. GS knockout technology is a well-established industry standard and is recognized by regulators as suitable for the manufacture of biologics.
CHOSOURCE works well with a diverse collection of vector strategies, from the standard random integration, with either both co-transfection of single promoter vectors or just using a single dual-promoter vectors, to transposase-mediated integration, which achieves very high product yields without substantial process optimization exercises. Horizon can provide access to vectors or recommend different options for those customers that don’t have internal vector technologies or have specific expression needs.
To facilitate customer success, Horizon provides comprehensive protocols with optimized instructions for every step from transfection to clone selection, to fed-batch overgrowth culture, and cryopreservation. A cell line history package is also provided to support regulatory submission with a fully documented traceable history of the R&D and manufacture of the CHOSOURCE cell line. CHOSOURCE has been used in multiple regulatory submissions for clinical trials in the USA, Europe, China, Canada and Australia.
Horizon has extensive experience in diverse gene editing platforms, from rAAV to nuclease genome editing technologies such as CRISPR, and offers gene-editing services including single and multiple-gene knockouts, knock-ins, point mutations, and the introduction of landing-pads for site-specific gene integration or other applications. Horizon offers these modifications in its proprietary CHOSOURCE cell line as well as cell lines provided by customers.
A New Approach to Freedom to Operate
Horizon’s CHOSOURCE cell line provides customers with a proven platform with a substantial degree of flexibility and freedom to operate, from compatibility with different vector systems, to different processes and media that can be used with our cell without any exclusivity restrictions.
Licensing advantages include:
- Freedom to choose any media, feeds, vectors, or to employ any CRO/CMO to suit the customer’s cell line development and manufacturing requirements.
- Freedom to operate, without any intellectual property or patent risks associated with using the CHOSOURCE platform for biotherapeutic production.
- Flexible licensing terms that facilitate risk-based decisions as required for a company’s business model and gives control over how the cell line is used.
- Future success is safeguarded with simple, royalty-free licensing to help maintain profitability throughout the product life cycle.
Since its introduction in 2016, CHOSOURCE been licensed to more than 85 companies worldwide with 10 confirmed clinical regulatory submissions (INDs/CTAs). CHOSOURCE has been used to produce multiple protein therapeutic architectures, from standard monoclonal antibodies, bi- and multi-specific antibodies, Fc-fusion proteins, as well as other customer products.
Case Studies
I recently viewed a webinar series on GEN that featured the use of CHOSOURCE across different therapeutic indications as well as application types. The webinar was a valuable resource as it demonstrated the various kinds of challenges that are being addressed by using CHOSOURCE. I have summarized the key takeaways below and provided links to view the webinars in full.
Cell Lines for Optimizing Biotherapeutic Production Workflows
Presented by Allyn Spear, PhD, Principle Research Scientist, Discovery Research, Elanco Animal Health
In this webinar, Dr. Allyn Spear shares Elanco Animal Health’s experience implementing the CHOSOURCE GS knockout cell line in building a biotherapeutics research program in animal health. He shares details about their mammalian-based process and walked through their production workflow. Specifically, he shared their process for evaluating growth conditions, transfection methods, clonal cell line development, and productivity assessments.
Key data takeaways:
- The CHOSOURCE cell line was very robust and showed good cell culture growth properties, with doubling times of 18-20 hours in standard shake flasks.
- Selection was straightforward with standard Glutamine Synthase/MSX selection allowing a primary turnaround pool in 14-28 days. Other selection systems based on puromycin selection were also used (adding glutamine to the media) allowing 5-12 day turnaround time for pools.Transposase-based vector integration was used with CHOSOURCE with very good productivity results. Routinely obtain stable pool expression of 1-3 g/L on a variety of molecule types – monoclonal antibodies, Fc-fusion and other proteins.
- After single cell cloning, the productivity of different clones was assessed in 24-deep well plates and 125mL shake-flask. In a 14-day fed batch run, Elanco found comparable productivity.
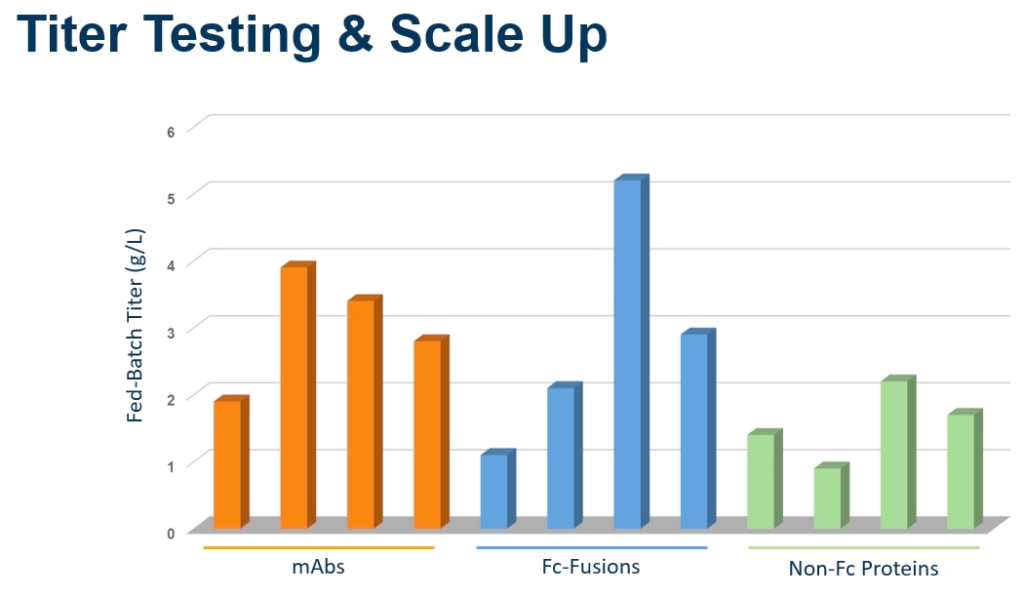
Dr. Spear commented that the CHOSOURCE system is wonderful at generating high producing pools and high producing cell lines early in the research path. Dr. Spear went on to say that the benefit of working in animal health is that you can move into target species to conduct PK, immunogenicity and even some efficacy studies early in research. Elanco uses 1.6 L Thompson Optimum Growth Flasks to support their material needs for these studies. Thus, they can achieve high volume in a small footprint. Dr. Spear closed by sharing titer performance for a fed-batch run across different molecule types (Figure 1).
Are you Stable or Transient? – Optimize your Biotherapeutic Cell Line Workflows
Presented by Michael Cullivan, Associate Scientist, Jounce Therapeutics
In this webinar, Mr. Cullivan explained how Jounce bridged the gap between their protein production group and their cell line development group by integrating the CHOSOURCE platform across different stages of the company’s drug development workflow. He also presented data on transient and stable transfections of CHO cell lines for various production purposes.
Mr. Cullivan and the drug development team began looking at whether transiently transfecting the Horizon CHOSOURCE cell line could help produce material for later stages in a shorter time frame. Previously, the protein production group was using ExpiCHO and the cell line development team was using Horizon CHOSOURCE. Jounce Therapeutics goals were (i) to achieve more than 90% monomer content post primary purification, (ii) adequate titer for their needs, (iii) a repeatable process across multiple runs, molecules and scales and (iv) to streamline process for current protein production group workflow. The team implemented a feed and enhancer strategy to improve titer.
Key takeaways:
- Transient CHOSOURCE cell line produced high purity material with low aggregation with more G1F and less Man 5 than ExpiCHO.
- Transient CHOSOURCE cell line resulted in lower levels of Host Cell Protein (HCP) than ExpiCHO. The ExpiCHO process required an additional purification step.
- Co-transfecting with GS gene improved titers and viabilities vs. supplementing with glutamine.
- A closer examination of supplemental enhancers, specifically Valproic Acid (VPA) and Sodium Butyrate/Propionate, resulted in a streamlined feed strategy:
- Addition of VPA 3 hours post transfection
- Elimination of Sodium Butyrate/Propionate
- Reduction of total supplmental glucose from 5% to 3%
This optimized protocol resulted in higher viabilities and titers on Day 11. It was also simpler and led to longer production runs and higher titers, thus allowing them to meet all goals of their study.
Mr. Cullivan conlcuded that transiently transfecting CHO-GS cells can produce material for later stage studies in a short time frame. For their next study they will look at whether they can produce a stable pool to increase the efficiency and speed of transfer from the protein production group to cell line development.
Optimizing Biotherapeutic Cell Lines for Continuous Manufacturing
Presented by Jeffrey McGrew, PhD, Scientific Director, Just-Evotec Biologics
In this webinar, Dr. McGrew describes how Just-Evotec Biologics have used the CHOSOURCE GS knockout cell line to develop high producing cell lines rapidly. He shared several case studies that demonstrate the cell lines’ ability to achieve cell densities in the range of 100 million cells per mL in perfusion processes with high specific productivities. This enabled Just–Evotec Biologics introducing their new intensified low-cost manufacturing processes as part of their new manufacturing facility workflows.
To achieve high productivity at the facility, Dr. McGrew shared that they achieved high yield from using small bioreactors. They identified a number of important steps in this process. Firstly, was to design the correct molecule by engineering molecules with improved manufacturability properties and yield in their molecular design group.
Secondly, they relied on the highly productive CHOSOURCE cell line, in combination with different expression vectors and media, focusing most often in their own proprietary vector and media platforms.
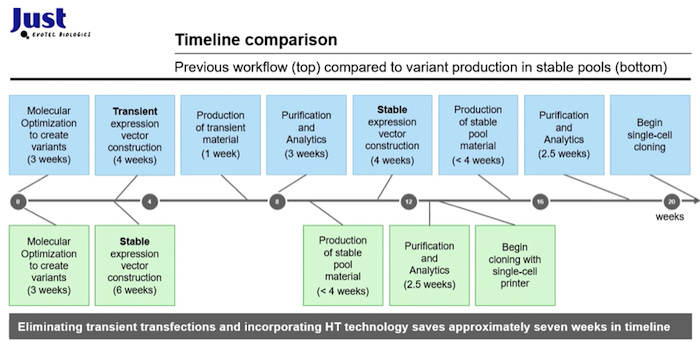
Next, Dr. McGrew presented interesting data , which compared the timeline for transient vs. variant production in stable pools. By eliminating transient expression and incorporating high throughput technologies, Just-Evotec Biologics was able to save approximately seven weeks in the timeline (Figure 2).
The team utilized different manufacturing strategies and Dr. McGrew presented data on several methods that they had tested.
Key data takeaways:
- Intensified fed-batch process culture of CHOSOURCE cell line A expressing a recombinant antibody at 2 L scale reached titer of ~15 g/L on day 13 with Viable Cell Density (VCD) up to ~140 million cells per mL and maintained viabilities greater than 90%.
- Continuous perfusion culture of CHOSOURCE cell line B expressing a recombinant antibody in perfusion culture reached 3 g/L /day on days 9-19. VCD was ~100 million cells per mL. Viability did drop below 80%, believed to be due to the molecule, other examples of the cell line in perfusion culture without low drop are available.
- Continuous perfusion culture CHOSOURCE cell line C expressing a recombinant antibody in perfusion culture reached 4 g/L/day with viability quite high and VCD at ~120 million cells per mL.
- Successful scale up of CHOSOURCE cell line D from 2 L to 500 L in intensified fed batch culture expressing a recombinant antibody reached 7 g/L on day 11 with excellent comparability for scale from 2 L to 500 L.
Summary
The three webinars highlight the great flexibility and robustness of the CHOSOURCE cell line which is able to:
- Provide optimal performance across different stages of biopharmaceutical discovery-development workflow
- Perform well and reliably in a wide variety of media and process workflows, and with a diverse expression vectors and transfection protocols
- Exhibit good performance across different manufacturing methodologies including standard fed batch, intensified fed batch and continuous manufacturing (perfusion) processes
- Give consistent performance with very diverse molecule architectures including standard recombinant antibodies, Fc-fusion proteins and other types of recombinant proteins
- Provide highly scalable processes
For specific study details, please view the webinars via the link below.
To learn more, please visit CHOSOURCE.