COVID-19 Therapeutics in Clinical Trials: The Immunomodulators
We scanned clinicaltrials.gov and found dozens of medications, world-wide, that have been in trial, currently are in trial, or are soliciting applicants for study. These medications include a include antivirals, antiviral cell mediators, immunomodulators, and other therapeutic classes. They are being evaluated singly, or in combinations, and usually across multiple clinical sites.
In a previous article, we presented many of the antiviral therapeutics that are being evaluated for COVID-19. In this article, we present several of the immunomodulating therapeutics.
Much of the mortality associated with COVID-19 is due to the development of acute respiratory distress syndrome (ARDS). ARDS is a type of respiratory failure characterized by immune activation, a cytokine storm, and widespread inflammation. The lungs are infiltrated by immune cells in an effort to control the virus.
Much of the damage to the lungs appears to be caused by inflammation, which can damage healthy tissue, rather than the virus itself. Reports have described an association between elevated cytokine markers and poor patient outcome (Ref, Ref). Thus, one possible approach to lessen patient mortality might be to control hyperinflammation through management of the immune response or the cytokine profile.
The exact composition of the cytokine storm in COVID-19 is still being defined by study. However, it appears that many key cytokines and chemokines are increased with COVID-19 infection, and many of these, in turn, are further increased with the severity of disease. The cytokines and chemokines include both Th1 and Th2-types and include both macrophage and CD4+ T-lymphocyte derived. Increased cytokines include IL-6, IL-1, TNF-α, IL-10, IL-2R, GCSF, IFN-γ, IP10, VEGF, and others (for more reading consult: Ref, Ref, Ref)
Another key aspect of COVID-19 is that the majority of patients with severe disease develop lymphopenia where the absolute number of total T-lymphocytes, CD4+ T cells, and CD8+ T cells can be reduced below the lower limit of normal (LLN). In addition, it has been noted that the frequency of some regulatory T cells (Tregs) and other immune cells can be reduced below LLN.
The exact cause of the lymphopenia is not clearly understood, and SARS-CoV-2 is not known to productively infect T-lymphocytes. However, the lymphopenia could possibly explain why some cytokines, such as IL-6 tend to reduce with increased severity of disease.
Given the complex nature of the immune response with COVID-19, there is little surprise that a diverse group of immunomodulators are being evaluated in clinical trials. We highlight many of them here.
Some of the immunomodulating medications in clinical trials for COVID-19
| Therapeutic | Therapeutic Class | Company | Notes |
|---|---|---|---|
| Small molecules | |||
| Corticosteroids | Anti-Inflammatory agent | Multiple | Hydrocortisone/Prednisone/Dexamethaxone/Budesonide |
| Progesterone/Estrogen | Sex hormone | Multiple | Steroid sex hormones with anti-inflammatory activity |
| Colchicine | Anti-mitotic, anti-inflamatory | Multiple | Gout therapy; antiproliferative; anti-inflammatory |
| CM4620 | Anti-inflammatory | Calcimedica | Investigational; CRAC channel inhibitor |
| Sirolimus (Rapamune) | Immunosupressant | Pfizer | Immunosuppressant; mTOR inhibitor |
| Ruxolitinib (Jakafi) | Anti-proliferative | Novartis/Incyte | Approved for myelofibrosis; JAK-1,2 inhibitor |
| Baricitinib (Olumiant) | Anti-rheumatic | Eli Lilly/Incyte | Approved for rhumatoid arthritis; JAK-1, 2 inhibitor |
| Recombinant proteins and monoclonals | |||
| CD24Fc | Immunosupressant | OncoImmune | Graft-vs-host disease; fortifies a inflammation checkpoint |
| Siltuximab (Sylvant) | Anti-neoplastic | EUSA Pharma | mAb; anti-IL-6; anti-neoplastic; anti-inflammatory |
| Tocilizumab (Actemra) | Anti-inflammatory | Genentech | mAb; anti-IL-6R (receptor); anti-rheumatic |
| Sarilumab (Kevzara) | Anti-inflammatory | Sanofi/Regeneron | mAB; anti-IL-6R (receptor) α chain; anti-rheumatic |
| Bevacizumab (Aviastin) | Angiogenesis Inhibitor | Genentech | mAB; anti-VEGF-1; angiogenesis inhibitor |
| Gimsilumab | Anti-inflammatory | Roivant Sciences | Investigational mAb; anti-GM-CSF; |
| Lenzilumab | Anti-inflammatory | Humanigen | Investigational mAb; anti-GM-CSF; |
| TJ003234 (TJM2) | Anti-inflammatory | I-Mab Biopharma | Investigational mAb; anti-GM-CSF; anti-rheumatic |
| BMS-986253 | Oncotherapeutic | BMS | Investigational mAb; anti-IL-8; solid tumor therapy |
| Canakinumab (Ilaris) | Anti-inflammatory | Novartis | mAb; anti-IL-1β, anti-inflammatory |
| Anakinra (Kineret) | Anti-rheumatic | Swedish Orphan Biovitrum | Recombinant IL-1 receptor agonist; E.coli manufactured |
| Emapalumab (Gamifant) | Anti-inflammatory | Sobi/Novimmune | mAb; anti-IFN-γ; for haemophagocytic lymphohistiocytosis (HLH) |
| LY3127804 | Angiogenesis Inhibitor | Eli Lilly | Investigational mAb; anti-angiopoietin 2; anti-inflamatory |
| Eculizumab (Soliris) | Complement inactivating agent | Alexion Pharmaceuticals | mAb; anti-terminal complement protein C5 |
| Ravulizumab (Ultomiris) | Complement inactivating agent | Alexion Pharmaceuticals | mAb; anti-terminal complement protein C5 |
| IFX-1 | Anti-inflammatory | InflaRx GmbH | Investigational mAB; anti-human complement C5a |
Small Molecule Immunomodulators
Hydrocortisone, (methyl) prednisone, dexamethasone, and inhaled budesonide are corticosteroids with immunosuppressant and anti-inflammatory properties. Inhaled budesonide is commonly used for the treatment of COPD.
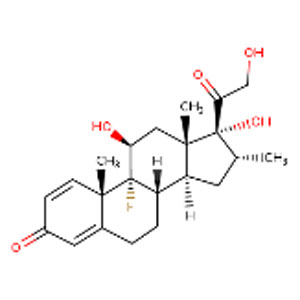
Corticosteroids were evaluated in dozens of studies of patients with SARS-CoV-1 and MERS-CoV for a possible benefit in reducing inflammation-induced lung injury. However, accumulated evidence from these studies suggests that corticosteroid treatment did not lessen mortality, but instead delayed viral clearance (Ref, Ref).
On June 16, the University of Oxford announced early results for part of the RECOVERY trial for COVID-19 taking place across 175 hospitals in the U.K. Their remarkable findings show that dexamethasone reduced deaths by one third in ventilated patents and about one fifth in those requiring oxygen alone. Based on these results, they estimate that 1 death would be prevented by treatment of around 8 ventilated patients or around 25 patients requiring oxygen alone. They observed no benefit among patients who did not need breathing support. If these studies are confirmed, dexamethasone would be the first therapeutic shown to improve survival from COVID-19.
Progesterone and estradiol are steroid sex hormones with anti-inflammatory properties and can modulate th1 and th2 immune responses. Progesterone is being evaluated in a proof-of-concept study and estradiol is being evaluated for male patients in another study. While it has been noted that male patients comprise a greater frequency severe of COVID-19 patents, there is no direct evidence, as yet, that sex hormones play a role in this bias.
Colchicine is used to treat gout and can reduce joint pain and swelling. It has also been used for Behcet’s syndrome, an inflammatory blood vessel condition, and the genetic disorder Familial Mediterranean Fever. Colchicine is inexpensive and has been used for centuries for inflammation. Despite its long history, there are concerns among some that colchicine may prove ineffective or may enhance the severity of COVID-19 due to its mechanistic actions.
CM4620 is a small molecule inhibitor of calcium release-activated calcium (CRAC) channels. CRAC channels are present on many cell types, including T-cells and mast cells and activation of these channels can play a role in inflammatory conditions. CM4620 is an investigational medication that is also being developed for patients with acute pancreatitis and accompanying SIRS.
Sirolimus (rapamycin) is an immunosuppressant that is primarily used in conjunction with cyclosporin and corticosteroids in the prevention of organ rejection following transplantation. It is indicated for use with kidney transplants. Sirolimus inhibits mTOR, which in turn, reduces activation and proliferation of T cells and B cells by reducing their sensitivity to several cytokines, including IL-2 and IL-15.
Ruxolitinib (Jakafi) is a antiproliferative that is used in the treatment of myelofibrosis, a myeloproliferative disorder of the bone marrow, and for polycythemia vera. It is also being developed for other applications such as inflammatory dermatitis. It is a Janus-associated kinase (JAK-1,2) inhibitor with immunosuppressant properties and is also used for graft-vs-host disease.
An small study of 20 severe COVID-19 patents showed an that ruxolitinib resulted in numerically faster clinical improvement and reduced levels of cytokines. However, the clinical improvement in this study was not robust enough to pass a test of statistical significance. A larger Phase III evaluation is planned.
Baricitinib (Olumiant) is another approved JAK-1,2 inhibitor that is approved for treatment of rheumatoid arthritis and is being developed for other applications. Baricitinib is being evaluated as a combination therapy with the remdesivir in a NIAID trial.
Recombinant proteins and monoclonal antibodies (mABs)
CD24-Fc is a developmental recombinant fusion protein that interacts with danger-associated molecular patterns (DAMPs) and sialic acid binding Ig-like lectin to attenuate immune activation and inflammation. It may also have application in graft-vs-host disease. A macaque primate model of AIDS using clonal-derived SIV showed that CD24-Fc reduced inflammation, lowered IL-6 and other markers, and protected 66% of animals against the clinical progression of inflammatory conditions linked to AIDS.
IL-6 is an important inflammatory marker that is elevated in COVID-19 patients. It has been suggested that IL-6 levels could serve as a marker for disease severity.
Siltuximab (Sylvant) is an mAB that binds directly to IL-6 to neutralize activity. It has antineoplastic properties and is indicated in the treatment of multicentric Castleman’s disease (MCD). Sarilumab may also have potential applications in some cancer therapies.
A ongoing preliminary study of 21 patients announced that 76% of the patients treated with sarilumab showed stabilized or improved disease symptoms at the interim analysis point. Moreover, IL-6 and CRP were reduced to normal levels within five days of treatment. This result of this study looks promising at this timepoint.
Tocilizumab (Actemra) and Sarilumab (Kevzara) are mABs that are indicated in the therapy of rheumatoid arthritis. Both tocilizumab and sarilumab inhibit IL-6 signaling via binding to the IL-6 receptor (Ref, Ref), rather than to IL-6 directly. The two mABs differ somewhat in their respective binding efficiency to the receptor and their clinical profile .
A preliminary study of 21 COVID patients treated with tocilizumab found a reduction of fever, an improvement of lymphopenia, and an improvement of CT scans in most patients. Two other studies of 57 patients and 53 patients treated with tocilizumab also have reported similar clinical improvements. However, a ongoing study of 400 patients treated with the similar mAB sarilumab have reported results that are not conclusive at the interim timepoint.
Bevacizumab (Avastin) targets VEGF-A and inhibits the process of angiogenesis (formation of new blood vessels). Bevacizumab is indicated for use in several cancers including, ovarian, cervical, renal cell carcinoma, glioblastoma, and some lung cancers.
VEGF-A is a potent factor for regulation of vascular permeability, and thus pulmonary edema. The rationale for use of Bevacizumab is that inhibition of VEGF may improve pneumonia, acute lung injury, and ARDS related to COVID-19.
Lenzilumab is an investigational mAB that targets GM-CSF. GM-CSF acts early in the inflammatory cascade and can induce production of other cytokines, including IL-6, IL-23, and TNF-α. Lenzilumab is being developed for use in chronic myelomonocytic leukemia (CMML) and juvenile myelomonocytic leukemia (JMML). In addition to receiving FDA approval for compassionate use in COVID-19, Lenzilumab is undergoing Phase III study for COVID-19.
Gimsilumab and TJ003234 (TJM2) are other anti-GM-CSF mABs under evaluation for COVID-19. Gimsilumab has completed Phase I studies for other indications, and Phase II studies are planned for COVID-19. The FDA approved TJ003234 in 2019 for clinical trials to treat rheumatoid arthritis and for COVID-19 trials in April, 2020.
BMS-986253 is an investigational mAB that targets IL-8 for use in the therapy of solid tumors. IL-8 can promote epithelial-mesenchymal transition (EMT), which then serves as an escape mechanism to reduce susceptibility to natural killer (NK) and T cell-mediated lysis. A Phase I trial showed decreases in serum IL-8 levels across all doses tested.
Canakinumab (Ilaris) is an anti-inflammatory mAB targeting IL-1β that is indicated for systemic juvenile idiopathic arthritis and periodic fever syndromes. The study plans to enroll 450 patients in Europe and the U.S.
Anakinra (Kineret) is a recombinant version of the human interleukin 1 receptor antagonist protein (IL-1RA) that also targets the activity of IL-1. IL-1RA counteracts and moderates the activity of IL-1. Anakinra is approved for cryopyrin-associated periodic syndromes (CAPS) and to reduce the symptom of rheumatoid arthritis.
A small uncontrolled study of 54 patients reported that anakinra reduced both the need for invasive mechanical ventilation and mortality among patients with severe forms of COVID-19-without serious side-effects.
Emapalumab (Gamifant) is an mAB that targets Interferon-γ (IFN-γ). It was approved in the U.S. in 2018 for the treatment of primary haemophagocytic lymphohistiocytosis (HLH), a rare genetic hyper-inflammatory condition characterized by the dysregulation of cytotoxic T cell and natural killer (NK) cell function, a cytokine storm, and the proliferation of lymphocytes and histiocytes.
Secondary HLH has a similar clinical, and cytokine profile to primary HLH except that is an acquired rather than a genetic disorder. There have been ongoing considerations that the cytokine storm and pathology in COVID-19 may be similar to that of HLH (Ref. Ref) and it is currently unknown if emapalumab will be beneficial to COVID-19 patients.
LY3127804 is an investigational mAB against angiopoietin 2 (Ang2) which may have application for treatment of some cancers. Increased levels of Ang2 promote tumor angiogenesis, metastasis, and inflammation. In concert with VEGF-A levels, Ang2 regulates vessel barrier integrity through endothelial cells. Ang-2 functions as an autocrine regulator of endothelial cell inflammatory responses.
Endothelial cells may contribute to ARDS by altering vessel barrier integrity, inducing vascular inflammation, and mediating cell infiltration (Ref, Ref). Phase II studies are planned for LY3127804.
Eculizumab (Soliris) is anti-complement C5 mAB that is indicated for paroxysmal pocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), among other indications. Eculizumab inhibits the complement pathway by binding to C5 and preventing the cleavage of C5 to C5a and C5b. Inhibition of cleavage, in turn, prevents the formation of the membrane attack complex (MAC) (C5b–C9), one of the most basic components of the innate immune system. It also prevents the generation of the C5a component which is a potent inducer of inflammation.
A small off-label study of 4 patients reported that patients treated with eculizumab, along with other therapeutics, had reduced levels of inflammatory markers. Mean C reactive protein levels dropped from 14.6 mg/dl to 3.5 mg/dl. Alexion has stated there is no evidence of the efficacy of eculizumab for COVID-19, and it is currently being offered through expanded access.
Ravulizumab is a newer anti-C5 mAB produced by Alexion that has been reported as non-inferior for treatment of PNH. Ravulizumab is a version of eculizumab that has been reengineered for longer lasting effect. Ravulizumab is undergoing Phase III studies for COVID-19.
IFX-1 is a developmental mAB designed to inhibit C5a. The drug is not believed to impact the formation of MAC (C5b-9). A small Phase II study with 15 treated patients showed no differences between in the oxygenation index in treated patients at day 5. However, they did observe a positive trend toward improvement of 28-day mortality. Phase III trials are under consideration.
Summary and Perspectives
Our survey shows a that a wide variety of repurposed immunomodulators are under evaluation in the effort to lessen the suffering and mortality of COVID-19. Many of these therapeutics mediate individual components the immune response rather than leading to broad immune suppression. We may find that modulators that can suppress the hyperinflammatory host response without significantly affecting viral clearance may result in the most benefit.
Since COVID-19 is a new disease that is not completely understood, it is entirely possible that some of these medications may prove detrimental to patient outcome. These therapeutics will need to be carefully accessed in clinic for possible harm. Patients are variable in response to any therapy and COVID-19 patients with preexisting inflammatory conditions may present another variable in therapeutic outcome.
We also note the recent report that treatment with dexamethasone can lessen mortality. Indeed, this finding serves as an initial proof-of-concept that immunomodulation can prove beneficial in the management of COVID-19.