CTS Detachable Dynabeads CD4 and CD8 Enable Flexible High-purity Isolation and Recovery of Target T Cells
As the field of cell therapy has evolved, our understanding of the interplay between CD8+ cytotoxic T cells and CD4+ helper T cells has changed. Although many therapeutic strategies initially focused on CD8+ T cells, trends evolved to include CD4+ T cells, either separately or in combination with CD8+ cells [1]. For example, some therapy workflows may aim to target predefined CD4:CD8 ratios that require isolation of single T cell subsets in parallel. Others may want to isolate single subsets of CD4+ or CD8+ T cells, while some therapies require isolation of all T cells.
Regardless of the specific process, clinical-phase cell therapy workflows targeting CD4+ T cells, CD8+ T cells, or both require CGMP-compliant materials and systems capable of consistently delivering the desired high-quality target T cells. Gibco™ CTS™ Detachable Dynabeads™ CD4 and Gibco™ CTS™ Detachable Dynabeads™ CD8 help support the clinical T cell therapy. industry’s growing need for CGMP-compliant solutions for isolating T cell subsets in the final therapy. Additionally, the use of the Gibco™ CTS™ DynaCellect™ Cell Isolation Kit with the automated, closed, and scalable Gibco™ CTS™ DynaCellect™ Magnetic Separation System further supports consistent delivery of high-quality, pure T cell therapeutics.
To evaluate the performance capabilities of the CTS Detachable Dynabeads CD4 and CD8, separate experiments were conducted with each product to specifically isolate either CD4+ or CD8+ target T cell subsets using the CTS DynaCellect system. Additionally, similar experiments were conducted to evaluate the combined use of the CTS Detachable Dynabeads CD4 and CD8 to simultaneously isolate CD4+ and CD8+ target T cells. These experiments were conducted with assessments of cell purity, viability, and recovery. In addition, the CD4:CD8 ratios were evaluated in the combined CD4+ and CD8+ isolations.
Materials and methods
Cells
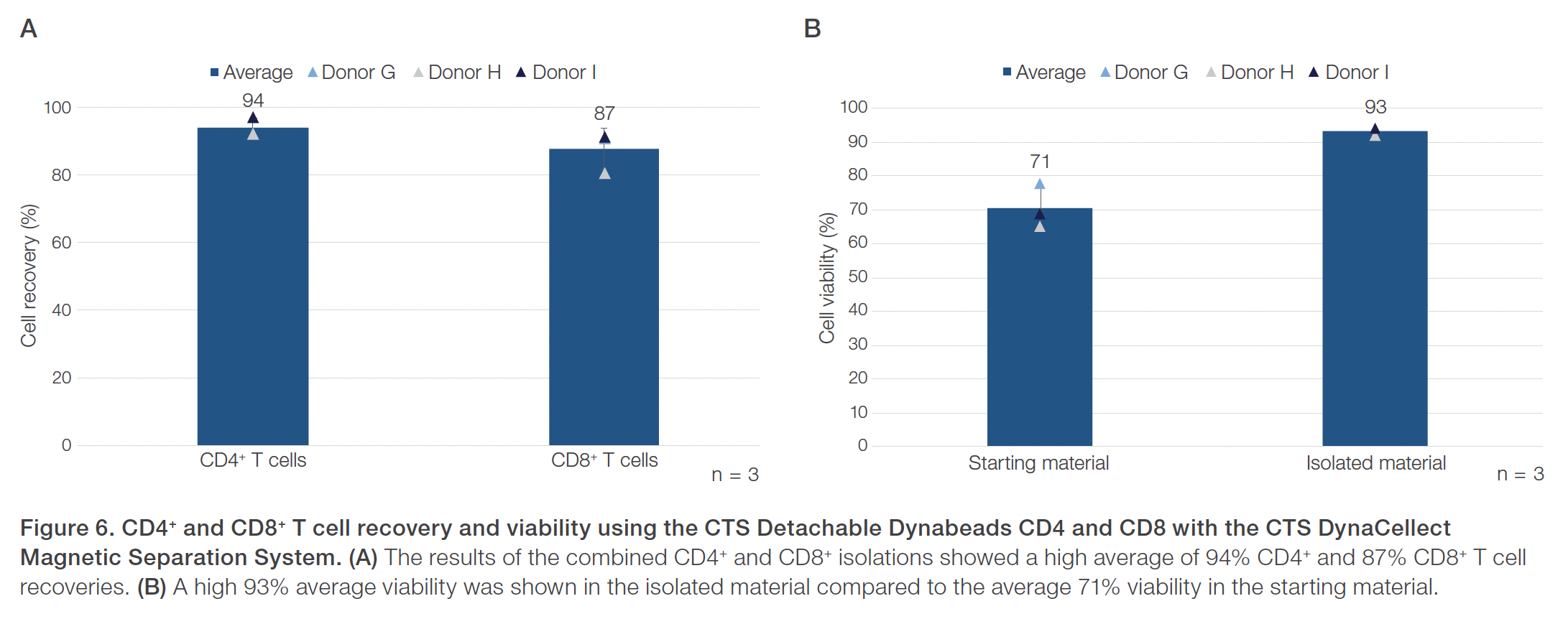
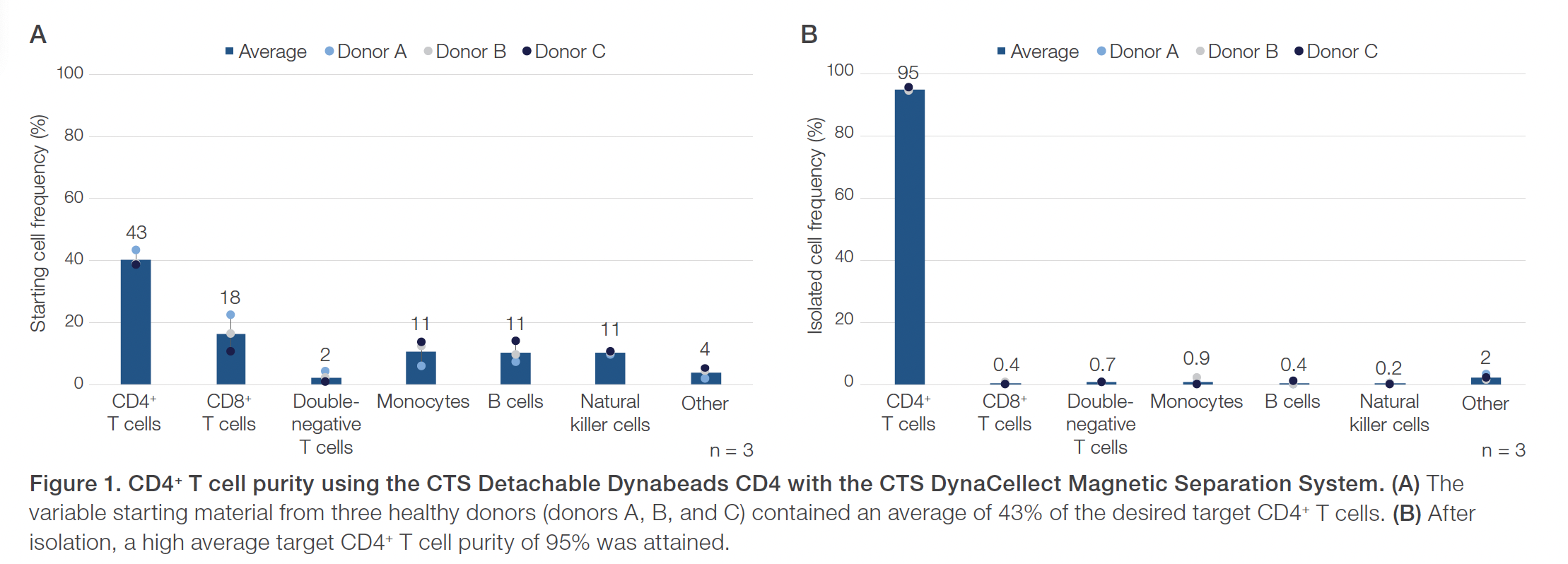
Peripheral blood mononuclear cells (PBMCs) from three healthy donors were used for separate CD4+ isolation experiments (donors A–C). Six other healthy donors were used for separate CD8+ isolation experiments (donors D–F) and the combined CD4+ and CD8+ experiments (donors G–I). Donor PBMCs from frozen leukopaks were recovered without washing and diluted 1:1 in a prepared buffer with Gibco™ CTS™ DPBS (Cat. No. A1285601) and 1% human serum albumin (HSA). The starting cell material was evaluated for cell count, viability, and purity. For the combined CD4+ and CD8+ isolations, the starting material was evaluated for CD4:CD8 ratios.
Isolation and release
In the separate CD4+ and CD8+ isolations, 4.0 x 108 target CD4+ or CD8+ cells were input based on the Certificate of Analysis of the starting material. In the combined CD4+ and CD8+ isolations, a quarter leukopak was input containing 6.0 x 108 to 14.0 x 108 target CD4+ cells and 4.0 x 108 to 9.0 x 108 target CD8+ cells. Cells were isolated with CTS Detachable Dynabeads CD4 (Cat. No. A56994), CTS Detachable Dynabeads CD8 (Cat. No. A56995), or both at a bead to target cell ratio of 4:1 using the CTS DynaCellect Magnetic Separation System (Cat. No. A55867) and the CTS DynaCellect Cell Isolation Kit (Cat. No. A52300). Isolated cells were actively released from the CTS Detachable Dynabeads CD4 and CD8 using Gibco™ CTS™ Detachable Dynabeads™ Release Buffer (Cat. No. A55883-03). After isolation and release, cells were evaluated for cell count, viability, purity, and recovery. Additionally, CD4:CD8 ratios were evaluated in the combined CD4+ and CD8+ isolations.
Performance criteria
In the separate and combined CD4+ and CD8+ isolations, viability and T cell purity were assessed in the starting material and the isolated cell material using the Invitrogen™ Attune™ NxT Flow Cytometer. In the separate CD4+ and CD8+ isolations, cell counts of the starting material and the isolated cell material were assessed using the Invitrogen™ Attune™ NxT Flow Cytometer, while in the combined CD4+ and CD8+ isolations, the NucleoCounter™ NC-200™ automated cell counter (ChemoMetec) was used. T cell recovery was calculated using the following equation: T cell recovery (%) = [(average isolated CD3+CD4+ or CD3+CD8+ cell count x volume)/(average starting CD3+CD4+ or CD3+CD8+ cell count x volume )] x 100.
Results
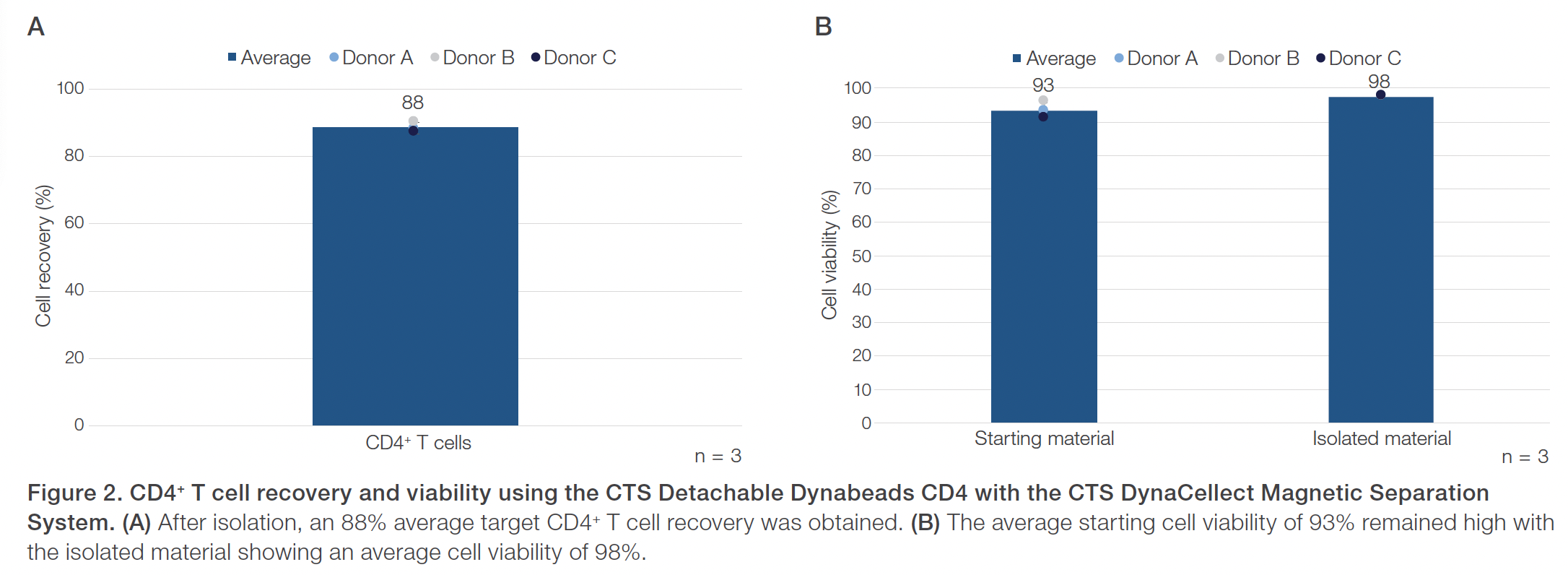
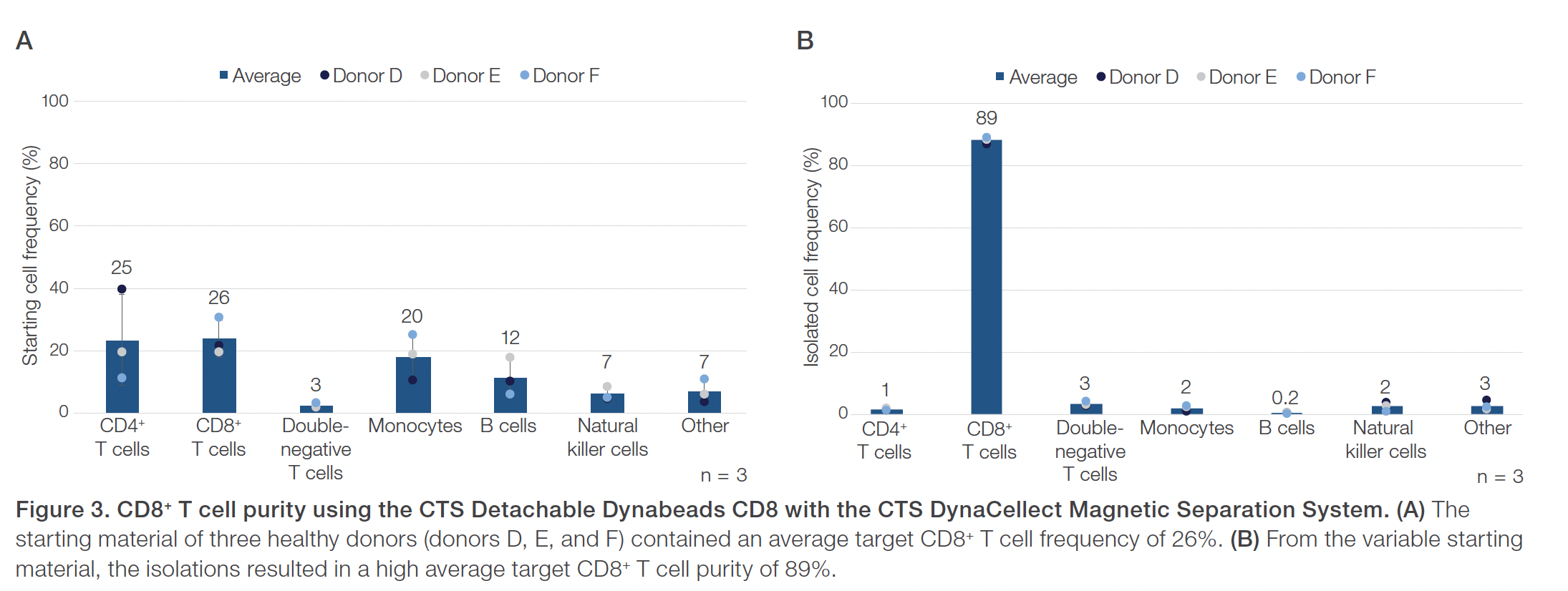
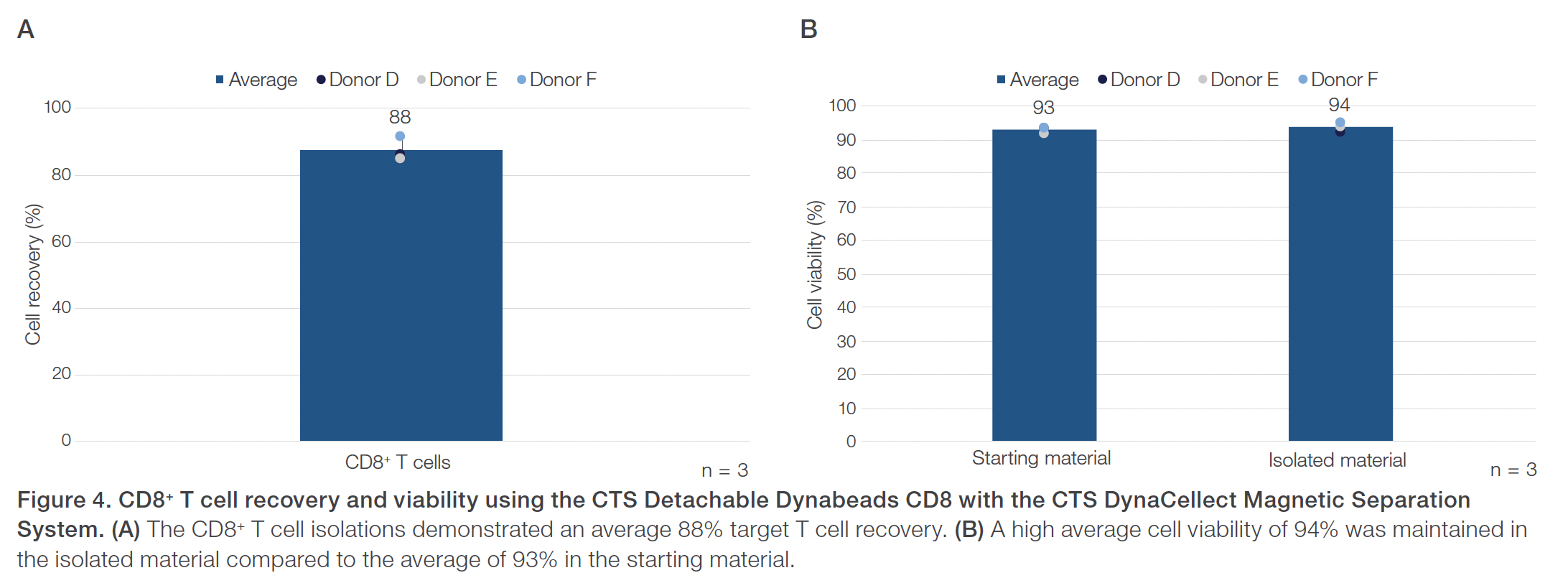
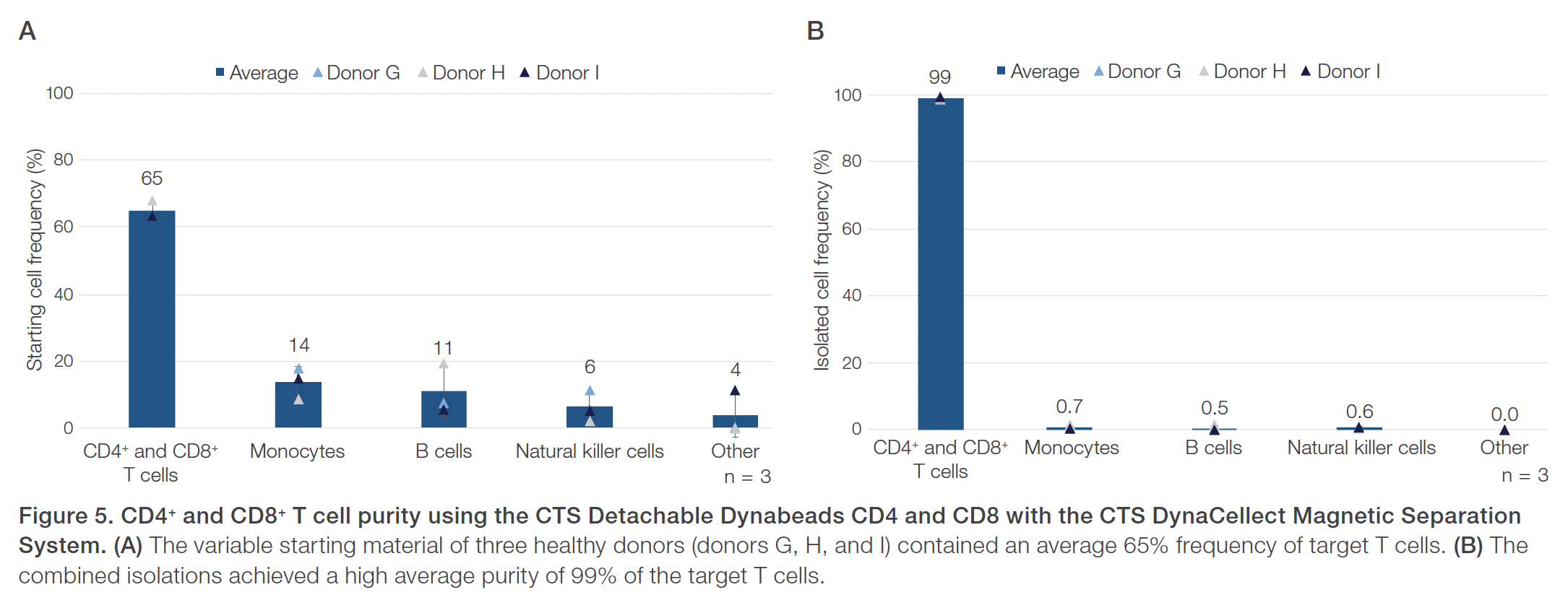
Evaluations of the CTS Detachable Dynabeads CD4 using the CTS DynaCellect system showed an average 95% CD4+ T cell purity compared to the average 43% CD4+ T cell purity in the starting material (Figure 1). The CD4+ isolation results also demonstrated an average 88% CD4+ T cell recovery and maintained a high 98% average cell viability (Figure 2). The results obtained using the CTS Detachable Dynabeads CD8 and CTS DynaCellect system showed an average 89% target CD8+ T cell purity from the average 26% CD8+ T cell purity in the starting material (Figure 3). The CD8 isolation results showed an average 88% target T cell recovery and 94% viability (Figure 4). Lastly, using the CTS Detachable Dynabeads CD4 and CD8 in combination to isolate CD4+ and CD8+ T cells resulted in obtaining an average 99% purity of target T cells (Figure 5). The combined isolations resulted in high average recoveries of 94% CD4+ and 87% CD8+ target T cells with 93% average cell viability (Figure 6). Additionally, in the combined isolations, the CD4:CD8 ratios were maintained from the starting to the isolated material (average CD4:CD8 of 1.6 before isolation and 1.7 after isolation; data not shown).
Conclusions
Overall, the experiments showed the CTS Detachable Dynabeads CD4 and CTS Detachable Dynabeads CD8 can be effectively used to consistently isolate target CD4+ or CD8+ T cell subsets, separately or together, at near 90–100% purity. The CTS Detachable Dynabeads CD4 and CD8 were also shown to provide consistent target cell recoveries near 90% or higher while maintaining 90% or higher cell viability. The CTS Detachable Dynabeads CD4 and CD8 demonstrated the flexibility to be used individually or in combination to effectively isolate their respective subset of target T cells. Additionally, the use of CTS Detachable Dynabeads CD4 and CD8 with the automated and closed CTS DynaCellect System further enables the consistent delivery of high-quality T cells post-isolation for both autologous and allogeneic therapies. As cell therapy manufacturing needs evolve, the CTS Detachable Dynabeads CD4 and CD8, along with the CTS DynaCellect Magnetic Separation System, can provide the process flexibility and scalability needed to support effective and consistent delivery of cell therapies for patients.
For more information, please download the full Application note at Flexible High-Purity Isolation and Recovery of CD8 and CD4 T-cells
Reference
1. Richardson JR et al. (2021) CD4+ T cells: multitasking cells in the duty of cancer
immunotherapy. Cancers 13(4):596. doi.org/10.3390/cancers13040596