A Defined Synthetic Growth Surface for Stable hiPSC Expansion
Introduction
hiPSCs cultivation can be challenging with lot-to-lot coating variations, tedious preparation and increased contamination risk. In particular, biological coatings may negatively impact downstream applications due to the presence of undefined extracellular matrix (ECM) components and growth factors. Another common struggle is decreased reproducibility of data due to an inherently complex and non-defined growth surface. To address these common challenges, Eppendorf has introduced a ready-to-use consumable with synthetic fibronectin-derived motifs to support stem cell attachment by mimicking the native ECM protein fibronectin. The FN1 motifs surface allows expansion of stem cells in various xeno-free media and other restrictive culture conditions. Thus, you can establish a completely defined culture system for your stem cell culture without any animal or human components.
A detailed expansion analysis was conducted and the results show that hiPSCs grown on FN1 motifs in xeno-free medium display their characteristic morphology. During their long-term expansion process across at least 20 successive passages, hiPSCs remain undifferentiated and retain all pluripotent stem cell-specific features including the trilineage differentiation potential as well as their genomic integrity.
Materials/Methods
Cryopreserved hiPSCs (SC102A-1, SBI, USA) were initially thawed and pre-cultivated on a Matrigel®-coated surface (Corning®, USA) in a xeno-free culture medium (Gibco® Essential 8™, Thermo Fisher Scientific®, USA). After 5 passages cells were seeded on CCCadvanced™ FN1 motifs surface (Eppendorf, Germany) using the classical clump passaging technique. The culture medium was exchanged daily until confluency level of interest. The hiPSC growth performance on FN1 motifs surface was compared with hiPSCs cultured in parallel on a Matrigel-coated surface in a biological feeder-free culture system. hiPSCs were cultured on FN1 motifs for 25 successive passages and monitored daily for colony morphology, confluence, and spontaneous differentiation. Doubling time, pluripotency marker expression, karyotype, and in vitro trilineage differentiation potential was analyzed to complete the characterization of hiPSCs during long-term expansion on FN1 motifs. Please refer to the Eppendorf Application Note 389 [1] for detailed information about material and methods of these experiments.
Results and Discussion
Morphology and proliferation
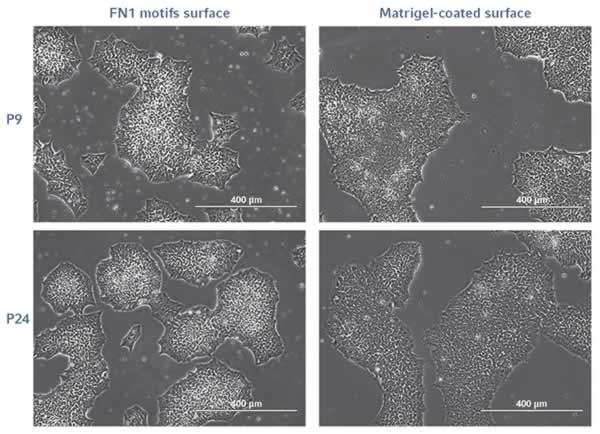
The FN1 motifs surface supports a robust long-term expansion of undifferentiated hiPSCs in xeno-free conditions. The cell morphology corresponded to the morphology expected in a feeder-free culture system (Figure 1) and remained stable across all 25 passages. An efficient and stable hiPSC doubling time was measured with an average of 24 h. Morphology and doubling time were similar to hiPSCs expanded on a Matrigel-coated surface. These findings show that you can expect a morphology and proliferation rate for your hiPSCs cultured on the synthetic FN1 motifs surface comparable to cells expanded in a feeder-free culture system.
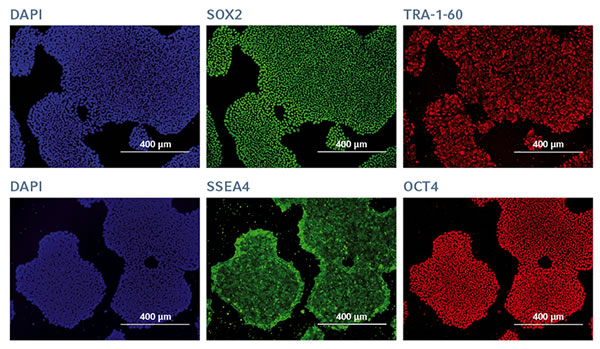
Functional pluripotency
An extensive characterization of the pluripotency phenotype confirmed the functional pluripotency of hiPSCs expanded on FN1 motifs. During the entire expansion process, very low levels of spontaneous cell differentiation were observed for hiPSCs (< 2% across the entire 6-well surface) confirmed by alkaline phosphatase staining. After 25 successive passages on FN1 motifs hiPSCs expressed pluripotent specific surface proteins TRA-1-60 and SSEA4, and self-renewal-associated nuclear transcription factor proteins SOX2 and OCT4 (Figure 2).
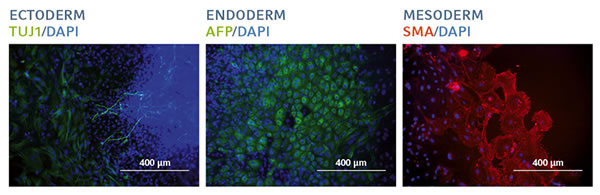
The image-based analysis was confirmed by flow cytometry analysis of the quantitative expression of key pluripotency-associated transcription factors: hiPSCs still exhibited a high level of Nanog, OCT3/4 and SOX2 expression (> 95% positive cells in the entire population), which was similar to that observed in hiPSCs expanded on the Matrigel-coated surface. The in vitro differentiation into cells of the three embryonic germ layers finally confirmed the functional pluripotency of hiPSCs after 20 passages on FN1 motifs surface (Figure 3).
Genomic stability
As long-term expansion in feeder-free conditions can lead to the occurrence of chromosomal abnormalities in pluripotent stem cells, it is very important to monitor their genomic stability. A G-banding karyotype analysis performed with hiPSCs obtained after 20 successive passages on FN1 motifs in xeno-free culture medium revealed a normal human karyotype (46XY) without chromosomal abnormalities. This confirmed the genomic stability of hiPSCs after long-term expansion on FN1 motifs surface.
Conclusions
The FN1 motifs provide a growth surface with high consistency for hiPSC culture. The FN1 motifs consumables enable the establishment of a defined culture system based on a synthetic surface, which mimics the fibronectin protein of the ECM. They combine convenience with reliable performance: the ready-to-use surface significantly reduces labor time and effort and ensures efficient long-term expansion of hiPSCs.
More about Eppendorf CCCadvancedTM FN1 motifs at www.eppendorf.com/ccc-advanced
Corresponding author: Nadine Mellies, mellies.n@eppendorf.de
Corning® and Matrigel® are registered trademarks of Corning, Inc., USA. Thermo Fisher Scientific® and Gibco® are registered trademarks of Thermo Fisher Scientific, Inc., USA. Essential 8™ is a trademark of Cellular Dynamics International Inc, USA.
Eppendorf® and the Eppendorf Brand Design are registered trademarks of Eppendorf AG, Germany. Eppendorf CCCadvanced™ is a trademark of Eppendorf AG, Germany.