Development of an Improved Poloxamer 188 – Optimized for Cell Culture Performance
Introduction
Poloxamer 188 is a surface-active non-ionic polymer used in cell culture media as shear protectant. With time it became a standard ingredient in cell culture media for commercial production processes. It was demonstrated to increase the robustness of mammalian cells to shear from sparging, which probably is the strongest contributor to the hydrodynamic stress in bioreactors.
- Reduces cell-bubble attachment
- May slightly alter bubble velocity and frequency
- Strengthens cell membranes
- Protective effect on cell surface receptors
- Lower energy level during bubble breakage
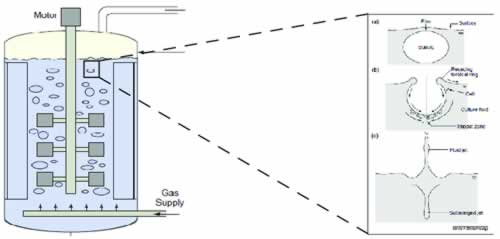
As mammalian cell culture technology improved, e.g., with process intensification through increasing cell densities and productivities in fed-batch and perfusion, issues with Poloxamer started to be reported. Several biopharmaceutical manufacturers reported unexpected loss of cell density and viability in their manufacturing operations which they could correlate to lot-to-lot variation in Poloxamer 188.
This variation became a major pain point in the biopharmaceutical industry and extensive investigations have been initiated to understand the source of lot-to-lot inconsistencies of Poloxamer 188.
The development and validation of proprietary analytical and biological methods based on a reference library of 100+ blinded customer and supplier samples has created the expertise to identify the critical properties of Poloxamer 188.
The new Poloxamer 188 EMPROVE® EXPERT cell culture optimized has been developed to achieve reliable quality and consistency providing shear stress protection for large scale cell culture processes.
Benefits
- Consistent quality — With our in-house developed methods we are able to predict the performance and ensure lot-to-lot consistency.
- Proven functionality — We test and certify shear protection on the Certificate of Analysis.
- Superior performance — Our product performs superior to lots across different suppliers, batches and quality grades.
- Reliable supply — Large manufacturing capacities ensure reliable supply for our customers.
- Full transparency — We provide full GMP documentation with our EMPROVE® dossiers.
Methods
To investigate the reasons for Poloxamer 188 failure, a series of tests and analyses was performed. With the input from industry leading biopharmaceutical manufacturers, various analytical methods have been assessed to identify correlations between physical and chemical properties and cell culture performance of Poloxamer 188. The goal was to find a procedure to reproducibly obtain Poloxamer 188 of reliable protective quality.
Applying a biological as well as an analytical assay, two orthogonal methods have been selected to demonstrate reproducible performance at larger production scales.
Development of a rapid cell-based shear protection assay
A cell-based method to assess and classify the shear protective effect was needed.
It has been decided to set up a cell based test based on vigorous shaking of CHO cells in baffled shake flasks. To increase throughput and shorten time to read-out, it was targeted to stress the cells for a couple of hours only. Shaking speed of the incubator was modified several times to increase selectivity of the assay. A chemically defined cell culture medium was used adding different Poloxamer 188 lots during media preparation. A parental CHO-S served as test cell line. Results could be reproduced with various CHO cell lines (e.g., CHOZN, CHO-K1).
Classification of lots using the rapid cell test
Based on a rapid test, three groups of material were arbitrarily classified for further investigation testing viable cell density (VCD) and viability.
It was observed that a first group of Poloxamer 188 samples had already decreased below 90% viability after 1 hour. These decreased further, with ongoing stress exposure, to values between 70% and 30% after 4 hours. The other groups were relatively close after 1 hour, but spread out over time. Only a few lots remained at very high viability close to 100%*. Control lots for either group had been defined.
This classification was then used to further study the chemical properties of these groups and identify common traits of good performing lots. In parallel, an improved assay was developed to better separate good against poor performing lots.
Development of an analytical method
In addition to the biological assay, analytical methods have been developed to compare the molecular weight distribution of different Poloxamer 188 lots. We identified high and low molecular weight impurities with size exclusion chromatography (SEC) as well as hydrophobic impurities with liquid chromatography mass spectrometry (LC-MS). Based on these results we have established an analytical method to reliably discriminate between good and poor performing lots.
Performance
Based on results discussed above, Poloxamer 188 properties that positively correlated with shear protective performance could be identified. Several lots of Poloxamer 188 have been produced and compared to the performance of commercially available material.
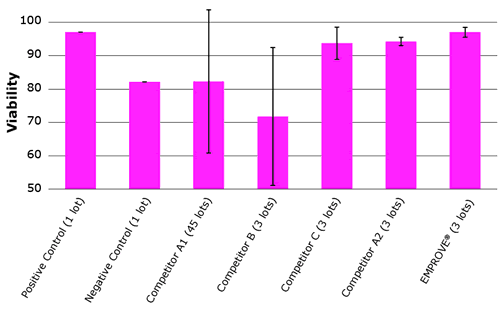
Figure 1 shows a benchmark comparison with other suppliers. The error bars indicate performance variations between tested lots. These show significant lot-to-lot variabilities between multiple lots of the same manufacturer. Each lot was tested in triplicate.
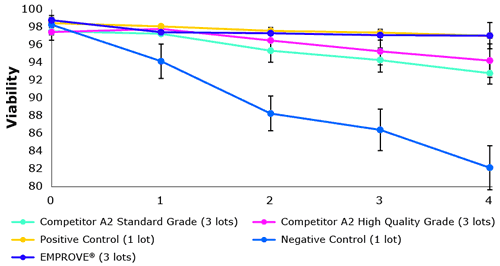
Performance issues in the biopharmaceutical industry were recognized by different manufacturers. This is why new quality grades have been developed. Figure 2 shows a benchmark comparison between different quality grades. Each lot was tested in triplicate.
High molecular weight components have proven to impact Poloxamer 188 performance negatively, especially when they are hydrophobic. Figure 3 shows molecular weight distribution of multiple lots of one competitor and the respective viability.
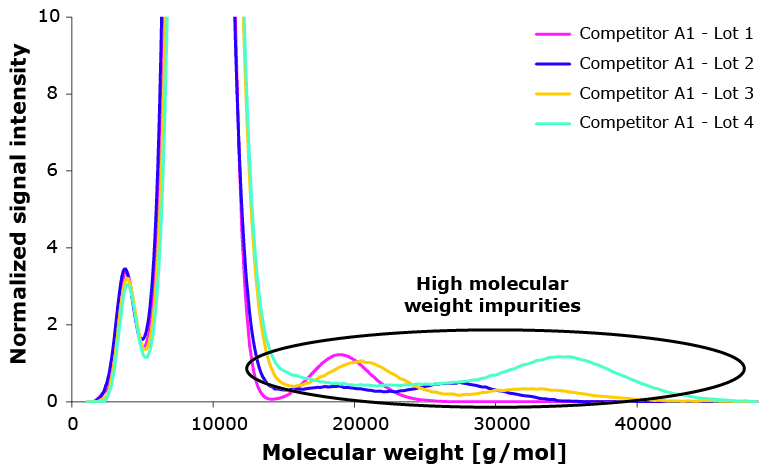
| Suspect Lot | Cell Viability |
|---|---|
| Lot 1 | 36% |
| Lot 1 | 31% |
| Lot 1 | 49% |
| Lot 1 | 70% |
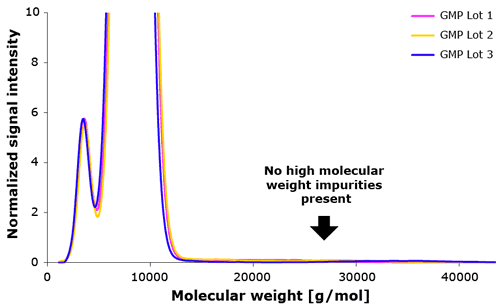
Based on the extensive knowledge gained from our studies we were able to develop Poloxamer 188 with non-detectable high molecular weight impurities together with our collaboration partner.
SEC data across multiple lots (Figure 4) shows that Poloxamer 188 EMPROVE® does not contain any high molecular weight impurities.
Consistency & Scalability
Biological drug manufacturers have stringent requirements with regard to process consistency and scalability.
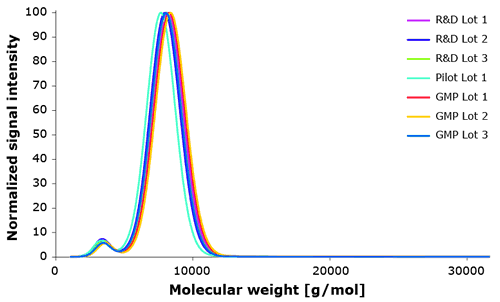
During product development all manufactured Poloxamer 188 lots were tested for lot-to-lot consistency by our analytical assay. Figure 5 demonstrates very consistent molecular weight distribution for small scale R&D (<10kg), non GMP (<1000 kg) and commercial scale GMP lots (~ 1000kg).
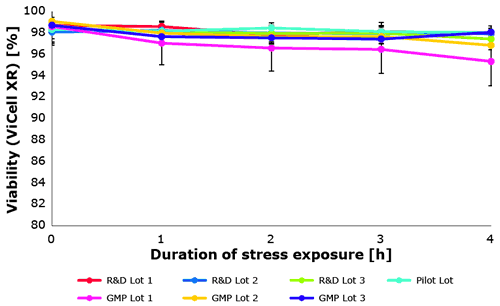
Also the biological assay proved a constant protective effect of the new Poloxamer 188 EMPROVE® over several production scales (Figure 6). Each lot was tested in triplicate.
Based on the extensive knowledge gained from our studies, we are able to reliably predict the performance of Poloxamer 188 lots. In collaboration with a trusted polymer manufacturer we developed a suitable process, which enables us to offer Poloxamer 188 of consistent quality and proven functionality for cell culture. Poloxamer 188 EMPROVE® EXPERT has been optimized for biopharmaceutical process from small to large scale production.
*The cell test implemented for standard product release differs from the test described in this section. Reason: A rapid test as above is a high throughput method of relatively low resolution.