
Direct Inoculum of Bioreactors with CHO Cells from Frozen Seed Bags to Eliminate Continual Seed Trains and improve Facility Utilization
Flexibility in cell culture manufacturing via a reduction in process duration can be a key strategy for maximizing facility utilization and facilitating the production of multiple therapeutics from a facility. A key bottleneck is the seed train, which can add weeks to the timeline of the production culture. Seeding production bioreactors with a direct, cryopreserved CHO cell inoculum could possibly eliminate the need for a lengthy, continuous seed train and provide other numerous benefits.
At this year’s Boston Biotech Week, two feasibility studies were presented that showed the use of a Frozen Seed Bag (FSB) for direct cell inoculum into a production bioreactor. Shahid Rameez of KBI Biopharma and Nikhil Ramsubramaniam of Merck presented separate studies demonstrating the use of frozen inoculum in fed-batch production bioreactors (KBI) or perfusion bioreactors (Merck).
KBI described a current state-of-the-art production process consisting of scale up from spinner flasks, through a bag bioreactor into stirred tank bioreactors – a 5-week process (see Figure 1). According to the study results presented, implementation of a direct frozen inoculum approach could potentially reduce a 5-week timeline to as little as 1.5 – 2 weeks. Such an improvement could vastly increase the productivity of current cell culture manufacturing facilities.
Figure 1:
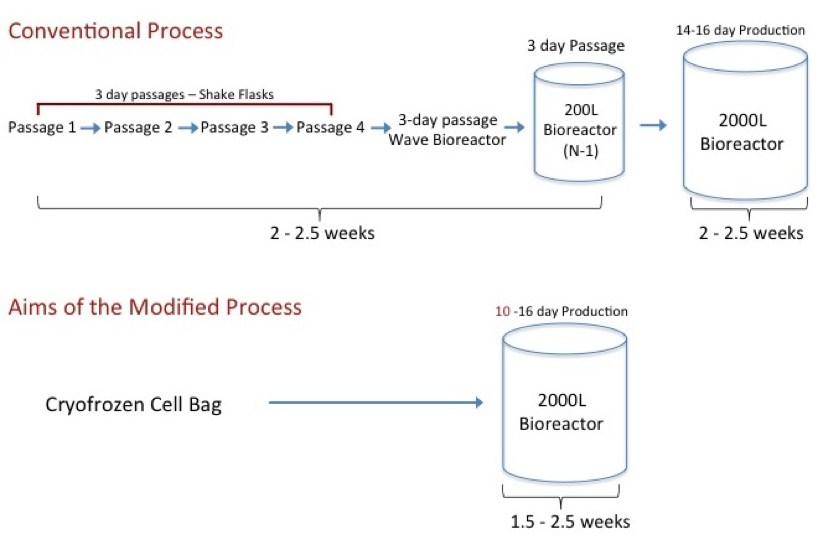
Other potential advantages in using an inoculum from a FSB include reduced contamination risk, improved consistency, the ability to seed at increased cell density to shorten the production bioreactor run itself, improved bioreactor performance, and improved integration of single use systems.
Merck presented on their seed train process, which used a perfusion bioreactor rather than a conventional seed train to produce concentrated, non-frozen cell seed for a production perfusion bioreactor. Merck’s main aims in the study were to improve consistency, eliminate the need for a continuous seed train, and to have the ability to increase cell seeding density to more quickly reach steady-state cell growth. Goals were to show feasibility for future utilization in a 500L production bioreactor.
The primary considerations by both groups were the properties and quality of the FSB. Both groups found that the FP-FLEX™ bags manufactured and offered by Charter Medical had the desired attributes and demonstrated the necessary consistency as well. Attributes included:
- Bag and tubing stable at frozen temperature (-196° C)
- Convenient welding of FP-FLEX™ tubing to C-flex® or PharMed® bioreactor tubing post-thaw
- Fits widely used freezing/storage cassettes
The concentrated cell seed was generated using perfusion bioreactors in both groups’ studies. KBI outlined a development approach which involved cultivating cells to high density prior to cryopreservation using two approaches: (1) ATF cell retention system, which separates spent medium from cells using a hollow fiber filter. (2) kSep® system (Sartorius Stedim Biotech), which harvests cells as a concentrated fluidized bed under a continuous flow of media using the balance of centrifugal and fluid flow forces. One advantage of the perfusion system was the ability to easily generate a concentrated cell suspension of up to 100 M cells/mL. 7 to 10 days were sufficient to produce the concentrated cell seed in the perfusion bioreactor.
FSBs containing 30 to 400 mL of cells at 20-50 M cells/mL were used in the study depending on the desired inoculum volume, the desired cell seeding density, and the size of the production bioreactor. Both groups used DMSO-based cryopreservatives. Cells were frozen by slow freezing or by flash freezing. Merck reported that rapid freezing produced the best results in their evaluation and FSB preparation required only 1 hour of time. Thawing of the frozen seed simply consisted of placing the bag in a warm water bath for a few minutes prior to inoculation.
Both groups found that direct inoculum of production scale bioreactors at the same cell density could successfully reproduce the same culture performance, productivity, and product quality, and product glycan profile as the conventional seed train approach (Figure 2-4). Optimization of cell seed volume, DMSO concentration may be required.
KBI also studied effect of increasing cell seeding density by 3-fold in the production bioreactor that could provide further benefit by reducing the runtime in the production bioreactor itself. However, KBI observed negative effects to cell growth and productivity, although product quality was not impacted, at 3-fold increased cell seeding density. One possibility is that carry-over DMSO cryopreservative is responsible for this negative effect. Further studies are planned to optimize the level of DMSO .
Figure 2: Evaluation of variability in FSBs using stirred tank bioreactors
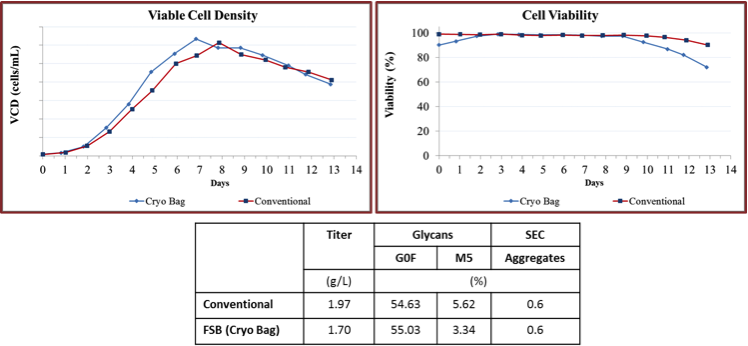
Figure 3: Evaluation of variability in FSBs using AMBR 250 fed-batch
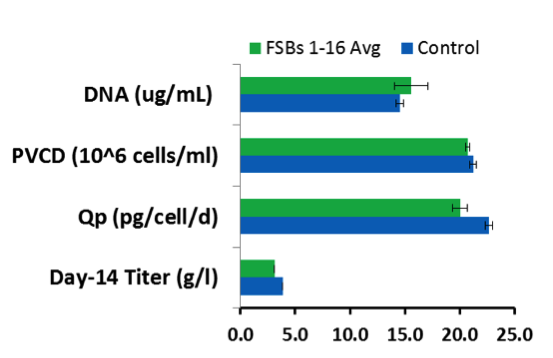
• Inoculation density: 0.5 *10 ^6 cells/ml
• Low inoculation density keeps DMSO in bioreactor below a certain threshold
Merck also reported an initial drop in cell viability when using a frozen inoculum in an AMBR 250 production system. This was corrected through an adjustment of dissolved oxygen, DMSO concentration and by reducing exposure time to the cryopreservative. DMSO carryover when cells were inoculated at low density did not significantly impact growth in a perfusion system.
Both KBI and Merck found little variation in product quality when using FSB compared to their traditional seed train process in a stirred tank bioreactors or 250 mL AMBR test system. Quality attributes included charge variants, aggregates, and glycan profile (Figures 1 and 4).
Figure 4: FSBs demonstrate low variation in quality compared to control
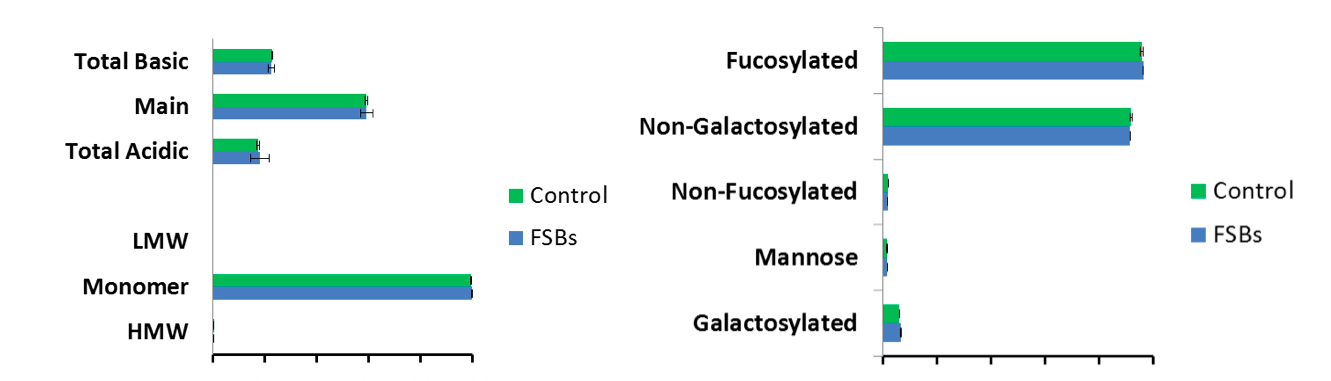
In summary, both groups presented promising feasibility studies supporting the direct inoculum of bioreactors with cryopreserved cells from a FSB. Similar cell culture performance, productivity, and similar product quality were seen. Future studies are planned to further optimize and expand on the benefits of this approach, which could result in more efficient facility utilization among other benefits.
Dr. Rameez talked about the path forward in terms of banking and characterization of FSBs. Their plans include:
- Improving the workflow for the banking of cells in the larger FSBs via controlled rate freezing methods.
- Developing assays for FSB characterization such as mitochondrial potential assays and mitochondrial transition pore assays.
- Evaluate FSBS for a variety of biopharmaceuticals and multiple cell lines.
Dr. Ramsubramaniam talked about the path forward in terms of scale up. Their plans include:
- Scaling up the size of the bag 4 fold.
- Achieving fill, thaw and freezing using lab-scale resources
- At frozen cell density of 50 M cells/ml, 5 bags will be required to inoculate 500 L
- Perfusion viable cell density, titer, and quality in AMBR 250 fed-batch should be similar to control at scale up.
I was fortunate to be able to ask Dr. Ramsubramaniam some questions about his presentation. Here is our interview.
Why did you decide to investigate the use of frozen seed bags for your seed train process?
The use of frozen seed bags combined with single use technologies can significantly reduce startup timeline for perfusion bioreactor experiments and at the same time help achieve steady state high cell density significantly faster than that achieved using seed train when a high cell density inoculation strategy is adopted.
How did you identify the key bag attributes you needed?
The key bag attributes were identified through screening experiments i.e thaw profile in small volume flasks of frozen seed bags made under different experimental conditions.
What did you find to be the most compelling data that this approach was worth moving forward with?
Similarity in growth characteristics, antibody expression (titer) and quality compared to seed train inoculation.
You mentioned in your presentation that you had to troubleshoot some areas. What advice would you give others who are interested in implementing the use of frozen seed bags?
Development of frozen seed bags for mabs would require competent perfusion expertise and getting there would be pivotal for successful implementation of this technology.