Ensuring FBS traceability using chemical fingerprinting
Fetal bovine serum (FBS) quality and traceability are very important, particularly in applications where the product is being used in biomanufacturing. While there has been a trend in much of the biopharmaceutical industry to move away from animal-derived products, the vaccine industry still uses FBS in vaccine production.
To address this need for absolute traceability, Oritain™ has developed a service to verify the fetal bovine serum origin based on the chemical fingerprint information that innately exists within the product. I am pleased to share the following guest blog that presents the use of Oritain’s chemical fingerprinting technology to scientifically verify the origin of FBS. GE Healthcare has collaborated with Oritain to align on an independent test-based traceability program to provide proof of the country of origin by testing actual serum samples to supplement the use of on paper documents.
I was fortunate to be able to interview two of the authors about the blog and have provided the transcript of the interview.
Using chemical fingerprinting to verify the origin of fetal bovine serum
A guest blog by Sam J. Lind (Ph.D., Science Commercialisation Director, Oritain), Robert Curry (MBA, Senior Product Manager for Serum, GE Healthcare), Cecilia Anneren (Ph.D., Global Product Marketing Manager, GE Healthcare), Cory Card (Principal Scientist, GE Healthcare), and Kiri McComb (Ph.D. Chief Technical Officer, Oritain)
Introduction
Many existing and new-generation protein drugs and vaccines required for human health and veterinary purposes rely on a cell culture manufacturing process. Whereas biological drugs are mostly produced under serum-free conditions, industrial vaccine production often requires FBS or serum derivatives. FBS is also used for cell culture in basic research and drug discovery. Whereas research users are largely concerned with the consistency of cell growth performance of serum from lot to lot, users in the industrial vaccine production segment are more interested in regulatory conformance and the lack of adventitious agents (1). Moving away from serum in general would be the best option for vaccine manufacturers, but it is not practical from an economic or regulatory perspective for most legacy vaccines. In addition, there are tens of thousands of different components in serum, whereas the most complex chemically defined media have between 100 and 150 components. Even today, the interactions between all of the serum components are not well understood, and for some cell lines, nothing performs as well as serum.
No matter what country it originates from, FBS will fortify cell culture media and support cell growth in a very similar manner. However, the variability between different serum batches remains a concern for the user. There is as much variability between serum batches within a country of origin as there is between countries of origin. For researchers, this is good news from a supply standpoint, as they can select the cheapest serum that can reliably reproduce results. The country of origin of the serum is of less importance. In contrast, for the vaccine manufacturers, the safety of the serum is of number one priority. Because sera are derived from animals, the presence of virus in serum is unavoidable, and different countries of origin present different levels of risk of specific virus contamination (2). Whereas certain bovine viruses occur worldwide, other bovine diseases display distinct geographic prevalence. Regarding bovine spongiform encephalopathy (BSE), for example, FBS from New Zealand and Australia has been promoted as “safer” than FBS of other origins, as these countries have isolated cattle herds and have never reported an outbreak. However, BSE has now been determined not to be transmitted in bovine blood or blood products, when appropriate slaughter practices are adhered to (2, 3).
The World Organization for Animal Health (Office International des Epizooties) is an international organization that monitors and provides updates on the presence of disease in different countries. As of January 2017, New Zealand, Australia, and the USA presented the lowest level of risk for infectious agent contamination in serum, including BSE, transmissible spongiform encephalopathy (TSE), and foot and mouth disease (FMD). For this reason, many vaccine manufacturers have one or more of these countries of origin specified in their manufacturing protocols that are registered with the FDA or international equivalents. These manufacturers have high demands on their serum suppliers to ensure that the country of origin is not only procuring accuracy from a regulatory standpoint, but also from an infectious agent standpoint (4).
While there are further steps that vaccine manufacturers can take to limit virus contamination risk, including gamma irradiation of the serum, these do not necessarily eliminate all risk of live virus being present in the serum. In fact, recent evidence suggests that some small non-enveloped viruses are not impacted by gamma irradiation (5). Whether these viruses are of concern is, however, an area of debate. In addition, some cell lines do not perform as well with irradiated serum, because gamma irradiation can have a negative impact on proteins present in the serum.
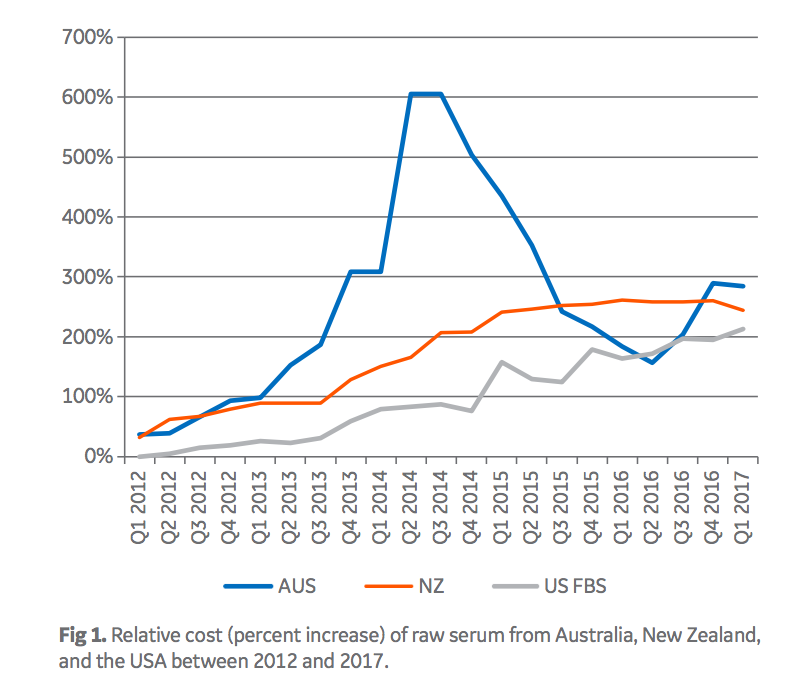
As a consequence of the large demand of sera from low-risk countries, there is a significant price premium paid for FBS from these origins. Today, Australian FBS, for example, is roughly nine times more expensive than Brazilian FBS. In addition to being driven by demand, prices for FBS are also driven by regulations and serum availability (1). Import-export regulations limit which sera can be brought into different countries. For example, under current regulatory conditions, Brazilian serum can be imported into the European Union and most Asian countries, but is barred from being imported into the USA. Moreover, as serum is a by-product of the meat industry, weather and other macro-economic factors impacting the beef industry can impact availability of FBS separately from the impact of demand. Limited supply in the USA and Australia recently drove those sera to record high pricing (Fig 1).
With the large price discrepancies between countries of origin, there is a financial incentive to cheat. By simply replacing the word Brazil on a bottle with the word Australia, the serum is instantly worth nine times more. From a customer standpoint, there is no way to tell the difference, as both Brazilian serum and Australian serum will have similar cell growth characteristics. However, the risk of virus contamination is different for the two products. Serum manufacturers and suppliers have a moral obligation to ensure that the country of origin is 100% accurate, and vaccine manufacturers are paying a substantial premium for low risk countries of origin to ensure that their vaccines that eventually end up in human beings have the lowest risk possible of doing harm.
The serum industry is largely self-regulated, and the International Serum Industry Association (ISIA) was not established until 2006. The ISIA has made major strides in standardizing terminology within the industry and establishing an independently audited traceability certification process that companies can qualify for. This traceability program has been very successful in curtailing the amount of fraudulent sera in the market place. Nevertheless, like most paper based traceability systems, it is limited in that someone does not willingly falsify documents.
Previously, when geographic origin could not be verified by testing, the buyer was left to rely only upon documentation for verification of origin. However, this has changed and traceability testing of animal products is now possible. Therefore, GE Healthcare decided to take the verification of serum origin one step further and has joined forces with Oritain as the provider, to introduce an independent traceability testing program based on Oritain’s proprietary scientific fingerprinting technology.
The tests are now performed to confirm country of origin of all lots of characterized and defined FBS from Australia, New Zealand, and the USA for GE Healthcare. This program supplements the ISIA certification and the use of paper documents as an additional assurance of the integrity and authenticity of the FBS.
How chemical fingerprinting works
It has been well established that soil geochemistry and climate patterns differ by geography. Plants take up chemicals from their environment in a ratio consistent with the local geography and form a “chemical fingerprint”. Likewise, ruminant animals, such as cattle, acquire a chemical fingerprint relating to their origin through consuming local food and water (Fig 2).
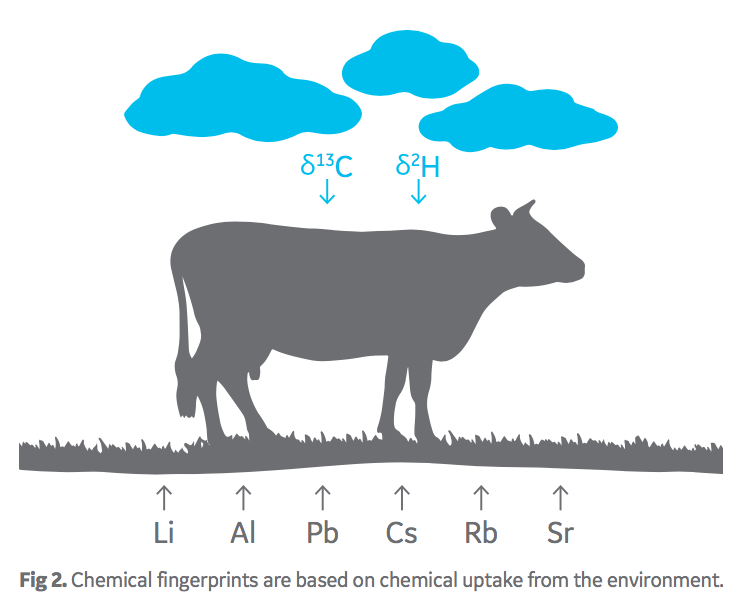
Oritain analyzes FBS products through chemical methods and statistical interpretation to determine the chemical fingerprint of origin. A comparison can be made with a FBS product acquired at any point in the supply chain, ensuring traceability claims are accurate.
Developing a method for chemical fingerprinting of sera
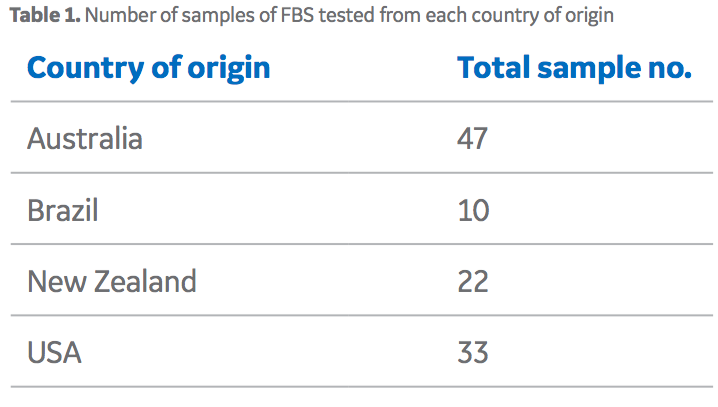
To develop the unique fingerprints of sera from different countries, GE provided samples from individual lots of HyClone™ FBS from four different countries of origin (Table 1). A 2 mL aliquot of each FBS sample was pipetted into a cleaned polytetrafluoroethylene (PTFE) digestion tube (exact sample mass was recorded) and 5 mL of quartz distilled HNO3 was added to the tube, which was then capped for a 1 h pre-digestion. Pressure was released from the tubes and samples were microwave-digested for 1 h. The digest was transferred to a new tube (and rinsed out with deionized water) and the solution evaporated to dryness in a graphite hot block. Thereafter, samples were re-dissolved in 10 mL of 2% HNO3 and sent for trace element analysis via inductively coupled plasma mass spectrometry (ICP-MS).
Results were corrected for blanks and digested mass to determine true elemental concentrations
in ppm. Data quality was validated at this level using a quality control (QC) material (characterized FBS standard) and a certified reference material (CRM, multielement water). Statistical analysis was carried out on a subset of analyzed elemental concentrations using Oritain’s proprietary statistical algorithm. Statistical data quality was checked for normality and any outlying data-points were identified and validated with analysis repeats. Sample set size was also checked with general methods to validate that the subset size was appropriate. Outputs included univariate and multivariate statistical analyses using a combination of general exploratory and discriminatory models.
Differentiation of sera from different origins
Figure 3 displays the graphical interpretation of the discriminant model developed to differentiate serum origins. Each data point represents the chemical fingerprint of an individual batch of serum. Most simply, points that cluster together have similar chemical fingerprints and are related in origin, while clusters that are separate from each other are of distinct origins. The values on the axis can be considered arbitrary but denote the variability of the chemical fingerprints.
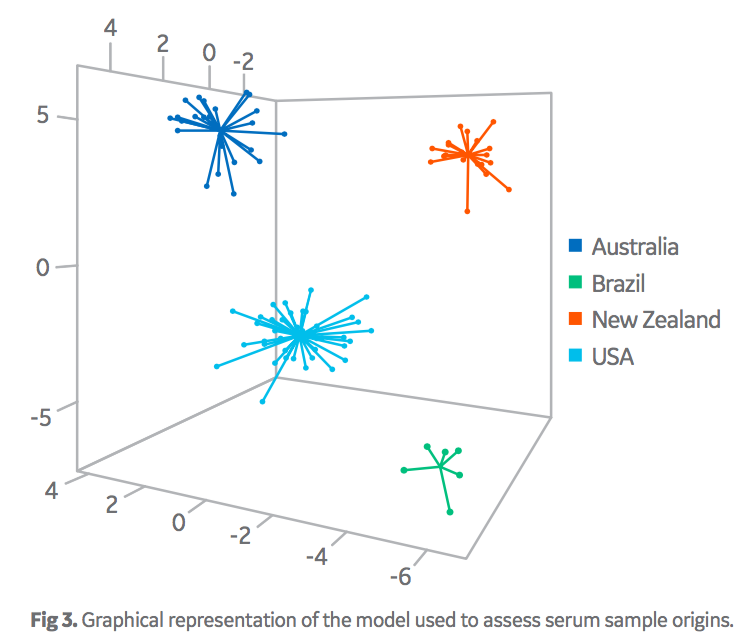
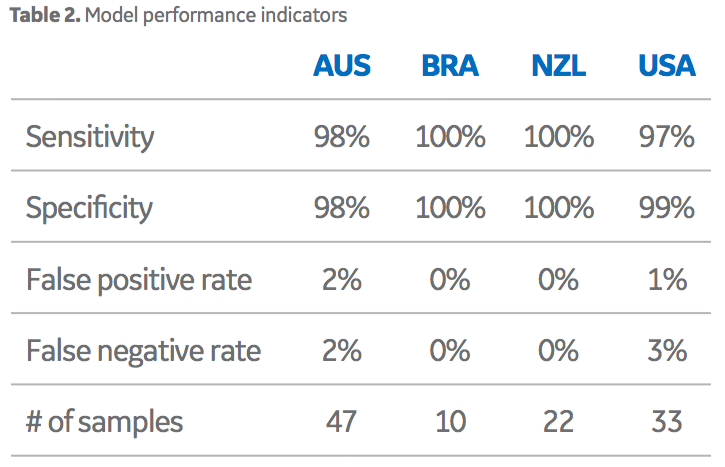
Importantly, the data clusters did not overlap each other across these three dimensions. Overall, this model performed well in terms of the ability to differentiate sera from four different origin groups based on their chemical fingerprints. Note, that samples sourced from Brazil, (high-volume, low- value origin) were easily discernible from the samples sourced from the other high-value origins. Overall performance is exemplified in the correct classification rate data in Table 2. As displayed, the false positive/negative rates are good (< 2% in most cases). Although this model is the main algorithm used by Oritain, there are several others, with similar performance parameters, that are used in concert to further minimize errors in commercial programs. In total, 12 independent statistical models are used by Oritain to interrogate the chemical fingerprint in relation to origin analysis.
Verifying the origin of HyClone FBS
The described model was also used to verify serum of a claimed origin. Baseline fingerprints of a number of HyClone FBS production lots were used to de ne a distribution for the distance of samples from the centroid of the multivariate cluster for a particular origin. Figure 4 shows a simulated example of a passed (origin: New Zealand) and a failed (origin: Brazil) result for a claimed origin of New Zealand. The reference fingerprint of the New Zealand samples are depicted as gray dots, with a 99% confidence interval of this data denoting the decision limit (blue line) between tested samples considered ‘consistent’ or ‘inconsistent’ (pass/fail) with the reference origin.
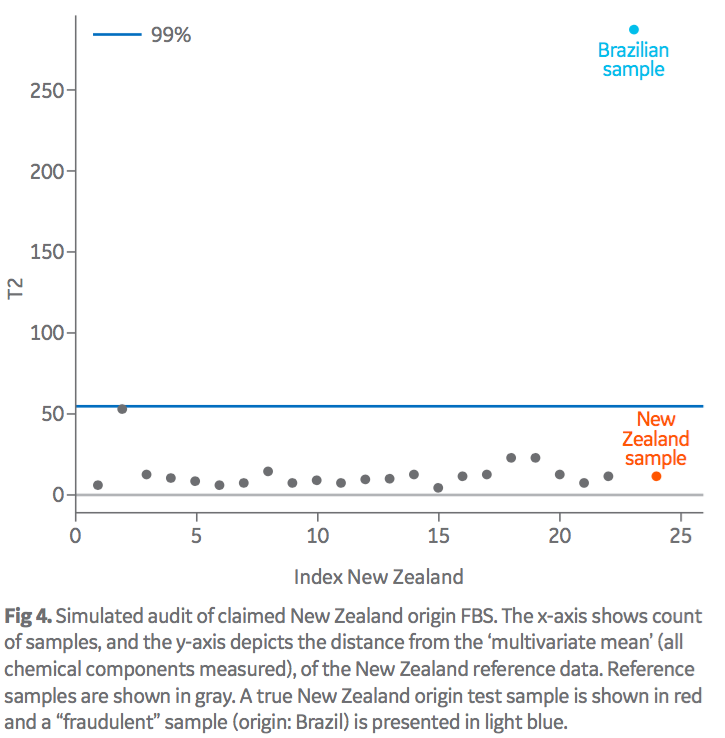
The effect of “origin blending” has also been investigated with binary mixes of differing origins. Initial studies show that it is also possible to identify “economic” levels of blending using this method (results not shown). A decision limit of a 99% confidence interval (blue line) is used to define the pass/fail limit for a tested sample.
Conclusion
Here, we describe the development of Oritain’s technology to identify the origin of sera. Using Oritain’s proprietary statistical algorithm, authenticity of a claimed serum origin can be verified and a fraudulently claimed origin identified. With the help of Oritain’s service, GE Healthcare takes a lead in the industry to chemically verify serum provenance and remains committed to the ISIA to roll out a larger industry wide-test based traceability program.
Interview with Sam Lind, Ph.D., Science Commercialization Director, Oritain & Robert Curry, MBA, Senior Product Manager for Serum, GE Healthcare
How was Oritain’s chemical fingerprinting technology developed? How did it evolve to be used in the serum industry?
Oritain’s Scientific Traceability method is based on the premise of forensic science. The technology was born out of the University of Otago by our founders and they saw it protecting producers from a range of industries against the risks of food fraud and substitution. Over time, the capabilities of the technology developed to cover products in the horticulture, honey, meat and dairy sectors. Fast-forward until recently, we undertook market research and investigation to ascertain if the technology could be applied to sera as it is a derivative of the meat industry. The results were positive and the fingerprints between origins is very distinct.
Why did GE Healthcare decide to employ the use of chemical fingerprinting in ensuring serum origin?
GE wanted to go beyond what is the standard in the industry. We want to show that GE is a leader in the serum market and that we have the best traceability program in the industry. This technology gives customers an extra level of assurance beyond standard paper traceability.
China in particular is a concern. In China we are confronted with counterfeit serum. Serum is a product goes into drugs that eventually go into humans. We want to give customers that highest level of assurance that what they buy is truly what is in the bottle. With this fingerprinting technology, questionable serum could even be tested and lot matched giving customers near unquestionable assurance that what they have purchased is genuine.
Why is it important to supplement traditional paper documentation with chemical fingerprinting technology?
The more a brand or a company does to protect their products and their brands the better. Paper based systems are certainly effective and have their place in the supply chain but are reliant on the parties to supply correct information. Also having a product based, independent testing solution like what Oritain specializes in, adds to the repertoire of tools that GE Healthcare uses to minimize supply chain risk
While paper based systems are effective, they only work to the extent that someone does not willingly falsify documents. While GE has a strict supplier quality management system, anything that we can do to expand that system provides additional assurances to customers.
It seems that this technology would be very useful to the serum industry as a whole. Do you think that Oritain’s fingerprinting technology will be adopted by the International Serum Industry Association? If so, how could it be implemented?
There is an agreement between Oritain and the ISIA and together we are working through how this technology can benefit the wider industry and associated stakeholders.
GE is actively engaged with the ISIA and promoting expanding this technology to the entire industry. Anything that can be done to improve the image of the serum industry is a positive for GE. We see ourselves as leaders in the industry and are happy that the entire industry want to adopt this technology.
What impact does chemical fingerprinting for serum origin have on regulatory interactions? For use of serum in vaccine manufacturing? For import/export regulations?
Although chemical fingerprinting is relatively new in the biopharmaceutical space (but very well receive) there is certainly a view that this technology has the ability to provide support in managing risk in the case of producers such as GE Healthcare and also for regulatory and customers. We see this space developing over the future as these additional stakeholders are included in the conversations and a suitable commercial framework put in place to solve for all parties.
Footnotes
-
1. Siegel, W. and Foster, L. Fetal bovine serum: the impact of geography. BioProcess J 12, 28-30 (2013).
-
2. Hawkes, P.W. Fetal bovine serum: geographic origin and regulatory relevance of viral contamination. Bioresources and Bioprocessing 2:34 (2015).
-
3. Hawkes, P.W. High-quality FBS may come from any authorized country of origin: adhering to the highest industry standards and regulatory requirements is essential. GEN Exclusives Online April 12 (2017).
-
4. Davis, D, Drake Hirschi S. Fetal bovine serum: what you should ask your supplier and why. BioProcess J 13, 19-21 (2014).
-
5. Cassart, J-P. Evolution of the virus detection technologies: threat or opportunity? Oral presentation at ISIA 2016, 11th Annual Meeting, Barcelona, Spain (2016).